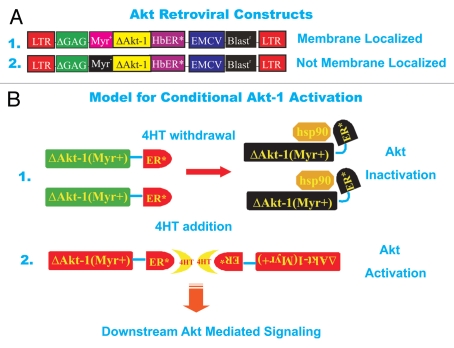
Figure 1.
Structures of Akt viruses used in this study and model for activation of Akt construct after 4HT treatment. (A) Conditional ΔAkt-1:ER*(Myr+) and (B) ΔAkt-1:ER*(Myr-) viruses which encode resistance to blasticidin. Fusion of two proteins yielded ΔAkt-1:ER*(Myr+) and (B) ΔAkt-1:ER*(Myr-). Human Akt-1 was modified by deleting amino acids 4 through 129, followed by replacement of D3 with glutamate and addition of a hemaggluinin subunit-1 (HA1) epitope to the carboxyl terminus to yield ΔAkt-1.17 The HA1 epitope consists of 17 amino acids with the sequence MAY PYD VPD YAS LGP GL. Amino acids YPY DVP DYA SL within this epitope correspond to amino acids 98 through 108 of HA1 from hemagglutinin-3/neuraminidase-2 (H3NS) stains of human influenza A virus. Deletion of amino acids 4 through 129 removes the pleckstrin homology (PH) domain from ΔAkt-1, which spans from amino acid 7 to amino acid 106. This domain mediates binding of Akt-1 to PI(4,5)P2 or PI(3,4)P3.16 The carboxyl terminal portion of ΔAkt-1:ER* is composed of a mutant murine ERα truncated at the N-terminus. This fragment is identical to amino acids 281 through 599 of murine ERα except that G525 is replaced by arginine. The portion of ERα fused to ΔAkt-1 contains the ligand binding domain, which spans from amino acid 306 to amino acid 556. However this ERα fragment lacks the DNA binding domain which spans from amino acid 184 to amino acid 266. Binding of 4HT to the ERα ligand binding domain of ΔAkt-1:ER* stimulates its Akt-1 activity. Replacement of G525 with arginine with the ERα fragment prevents binding of estrogen to ΔAkt-1:ER*. Replacement of the N-terminal methionine residue of ΔAkt-1:ER*with a myristoylation signal sequence yielded ΔAkt-1:ER*(Myr+). This myristoylation signal sequence corresponds to amino acids 1 though 15 of viral sarcoma (v-Src) from Schmidt-Ruppin subgroup A strain of Rous sarcoma virus. The v-Src myristoylation domain (Myr+) allows localization of the ΔAkt-1:ER*(Myr+) proteins to the lipid rich cell membrane. In contrast, ΔAkt-1:ER*(Myr-), has an alanine substitution in the v-Src Myr domain and is not myristoylated. Lack of myristoylation prevents ΔAkt-1:ER*(Myr-) from localizing to the plasma membrane. The genes encoding ΔAkt-1:ER*(Myr+) and ΔAkt-1:ER*(Myr-) were inserted into pWZLblast at the multiple cloning site (MCS) to yield ΔAkt-1:ER*(Myr+) ΔAkt-1:ER*(Myr-), respectively. The multiple cloning site of pWZLblast is situated between 5′ and 3′ Moloney Leukemia virus (MMLV) long-terminal repeats (LTRs). A MMLV retroviral packaging signal is located between the MMLV 5′LTR and the MCS. The gene encoding blasticidin S resistance from Bacillus cereus is positioned between the MCS and the MMLV 3′LTR. Blasticidin resistance is driven by an internal ribosomal entry site (IRES) from the Rueckert strain of encephalomyocarditis virus and encodes resistance to blasticidin in both Escherichia coli and mammalian cells. (B) Model for activation of conditional ΔAkt-1:ER*(Myr+) gene upon 4HT treatment. In the absence of 4HT, the ΔAkt-1:ER*(Myr+) proteins are associated with heat shock proteins such as Hsp90 and are inactive. In contrast, in the presence of 4HT, the ΔAkt-1:ER*(Myr+) proteins dimerize due to the binding of 4HT to the hormone binding domain of the modified estrogen receptor which binds 4HT 100-times more efficiently than β-estradiol (estrogen). This induces Akt activity and downstream signaling.