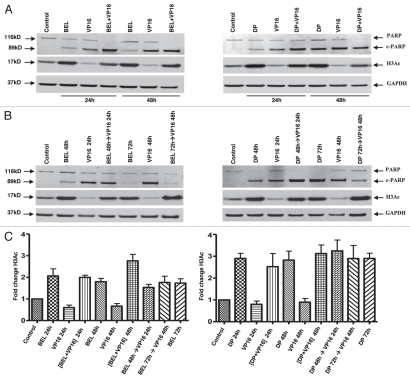
Figure 3.
PARP degradation but not H3 acetylation depends on the schedule of drug administration. H146 cells were treated with belinostat (0.3 µM) or romidepsin (1 ng/mL) and VP-16 (4 µM) for 24 h and 48 h as indicated. Protein gel blot shows degradation of PARP, appearance of cleaved PARP (89 kD), acetylated H3 (17 kD) and GAPDH used as a loading control (37 kD). (A) Effect of belinostat, romidepsin, VP-16 and simultaneous treatment. (B) Effect of belinostat, romidepsin, VP-16 or combination added sequentially, HDI→VP-16, with a 24-h delay (HDI for 48 h and VP-16 for 24 h). (C) H3 acetylation in H146 cells treated with belinostat (0.3 µM) or romidepsin (1 ng/mL) and VP-16 (4 µM). Bar graph represents quantitative analysis of H3 acetylation from immunoblots as in (A and B). Results are mean ± SD from three to four independent experiments. Data are presented for both belinostat (BEL) and romidepsin (DP) as indicated. For this and the remaining figures, the (+) symbol indicates simultaneous exposure, while the (→) symbol indicates sequential exposure.