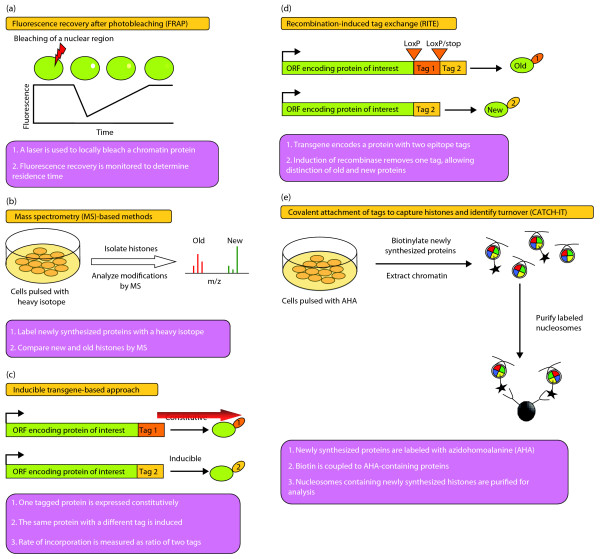
Figure 2.
Methods for investigating chromatin dynamics. (a) In fluorescence recovery after photobleaching (FRAP), a laser is used to bleach the fluorescence of a chromatin protein in a discrete region of the nucleus. The time required for the fluorescent protein to move back into the bleached region and restore fluorescence is proportional to the residence time of the protein on chromatin. The shorter the residence time of a protein, the faster fluorescence is recovered in the bleached region. (b) Mass spectrometry (MS) can be used to study the dynamics of post-translational modifications in the total histone pool by briefly labeling newly synthesized histones with a heavy isotope, such as 15N or 13C. Histone modifications can then be determined by MS for both the old and new histones, based on the mass difference between these two pools of histones. (c) In the inducible transgene-based approach, the protein to be assayed is expressed from two different transgenes. One transgene is expressed constitutively and carries an epitope tag while the other is inducible and carries a different epitope tag. Induction of the second transgene allows the measurement of binding kinetics by comparing the relative abundances of the two tags at a given genomic location. (d) Recombination-induced tag exchange (RITE) can be used to measure the dynamics of chromatin binding and nucleosome assembly by enabling old and new proteins expressed from the same transgene to be distinguished. This method uses a single transgene that encodes two different epitope tags. The first tag is flanked by loxP recombination sites and incorporates a stop codon, whereas the downstream tag is in-frame but comes after the stop codon. Normally, the protein encoded by the transgene has only the first tag, but after induction of Cre recombinase the first tag is removed from the transgene and now the encoded protein contains only the second tag. Dynamics of a RITE-tagged protein can be inferred by comparing the relative abundances of the old and new versions of the protein at a given genomic location. (e) Covalent attachment of tags to capture histones and identify turnover (CATCH-IT) can be used to estimate rates of disassembly and reassembly, or turnover, of native nucleosomes across the genome. In this method, newly synthesized proteins are labeled by treating cells with the methionine analog azidohomoalanine (AHA). Nuclei are isolated from AHA-treated cells and biotin is coupled to AHA-containing nuclear proteins through a reaction of the azide group of AHA with an alkyne linked to biotin. Chromatin is then digested to mononucleosomes, the nucleosomes are extracted, and nucleosomes containing biotinylated histones are purified via streptavidin. Stringent washes are used to remove H2A/H2B dimers and all other DNA-binding proteins from the purified nucleosomes. Microarray analysis or deep sequencing of the purified DNA allows the rates of nucleosome turnover across the genome to be estimated on the basis of the extent of newly synthesized H3/H4 dimer incorporation at each site.