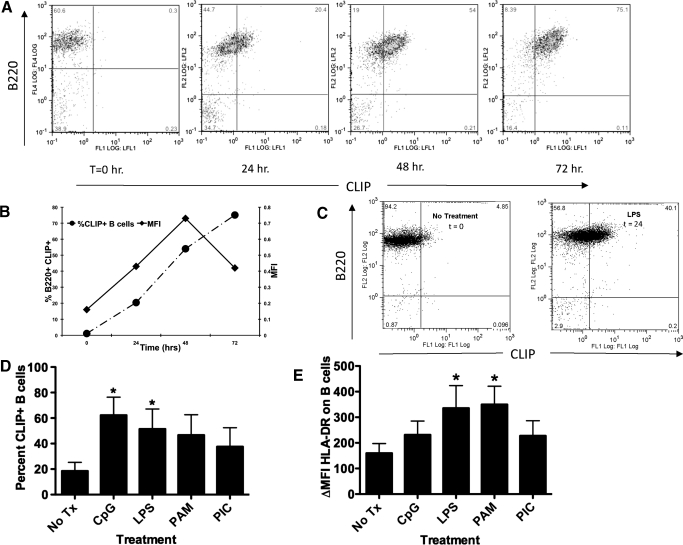
Figure 1. TLR activation of mouse spleen cells or human PBMCs results in ectopic CLIP in MHC class II on B cells.
(A) Immediately ex vivo, mouse splenocytes from B6.129 mice (H-2b) were treated with LPS for 24, 48, and 72 h as indicated. The cells were harvested at the indicated time-points and stained with 15G4-FITC and anti-mouse CLIP/I-Ab, as indicated on the x-axis, and PE-conjugated anti-mouse B220, antibody to the B cell molecule B220, y-axis, dually used to detect cell surface CLIP in the groove of I-Ab on B cells. The figure is representative of at least 4 experiments. FL1/2/4, Fluorescence 1/2/4; LFL½, laser fluorescence ½. (B) The percent CLIP+ B cells, left y-axis, dashed line with • (as determined by the staining method in A) was compared with the relative change in geometric MFI, right y-axis, solid line with ■, representing the increase in the amount of CLIP/I-Ab molecules per cell. (C) Purified resting B cells, isolated by magnetic bead separation (Miltenyi Biotec) from immediately ex vivo mouse splenocytes from B6.129 mice, were treated with LPS for 48 h. The cells were harvested and stained with 15G4-FITC, anti-mouse CLIP/I-Ab, as indicated on the x-axis, and PE-conjugated anti-mouse B220, antibody to the B cell molecule B220, y-axis, dually used to detect cell surface CLIP in the groove of I-Ab on B cells. (D) Human PBMC cells were stained using anti-human CLIP-FITC versus CD19-PE (values of isotype controls were subtracted from the specific stains, ΔMFI); nil vs. CpG, P = 0.06821; nil vs. LPS P = 0.0390; nil vs. PAM P = 0.0124. The data represent the percent of total PBMC that are CLIP+ B cells subsequent to each treatment. Each experiment represents at least 3 replicates. (E) Human PBMC from five donors were cultured for 24 hours with toll ligands (CpG-ODN, LPS, Pam3Cys, and Poly 1:C as indicated) and were stained with a pan anti-HLA-DR-FITC antibody (values of isotype controls were subtracted from the specific stains, ΔMF1).