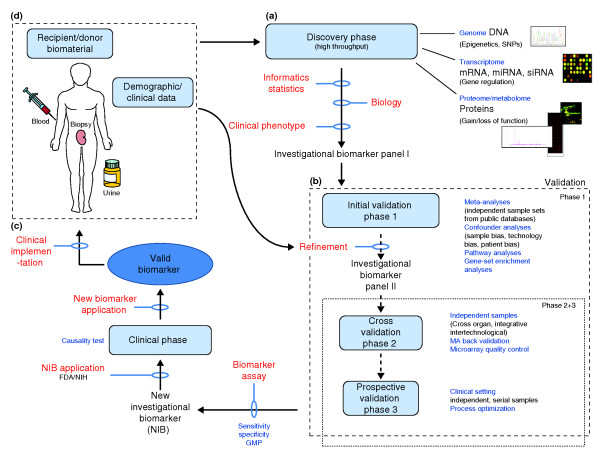
Figure 1.
Outline of the biomarker development process in the US from clinic to bench and back to clinic. As in drug development, the key phases are the discovery and validation phases, which involve complex FDA-regulated processes. (a) High-throughput, often in silico technologies are used to discover genomic, transcriptomic, proteomic or integrative investigational biomarkers, which are then (b) redefined in several validation phases using independent samples, technologies, and horizontal and vertical meta-analyses. (c) A clinically applicable biomarker assay based on good manufacturing practice (GMP) can be developed after prospective studies have confirmed the investigational biomarker. The FDA has to approve clinical studies, and only after successful completion and additional FDA regulation can the biomarker be considered valid and (d) be implemented into the clinic.