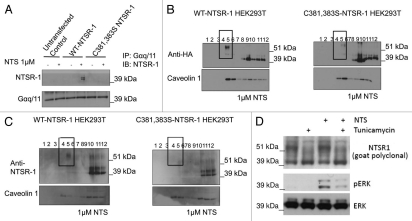
Figure 6.
NTSR-1 palmitoylation is required for optimal interaction with Gαq/11A within SMDs. (A) HEK293T cells transfected with WT-NTSR-1 and C381,383S NTSR-1 were serum-starved for 24 h before 15 min stimulation with 1 µM NTS. The cells were then lysed using TritonX-100 buffer and immunoprecipitated using anti-Gαq/11 antibody. The immunoprecipitates were immunoblotted with anti-NTSR-1. Representative blots from n = three separate experiments. (B and C) HEK293T cells transfected with HA-tagged (B) or non-HA-tagged (C) WT-NTSR-1 or C381,383S NTSR-1 were serumstarved for 24 h before 15 min stimulation with 1 µM NTS. The lysates were then subjected to detergent free-sucrose gradient fractionation and the fractions were immunoblotted with either anti-HA (B) or anti-NTSR-1 (C). All blots were re-probed with anti-caveolin-1 to assess equal loading as well as to identify caveolin-enriched SMDs. (D) HEK293T cells transfected with WT-NTSR-1 were pretreated with 1 µg/ml tunicamycin for 24 h before stimulation with 1 µM NTS for an additional 15 min. Representative blots from n = three separate experiments.