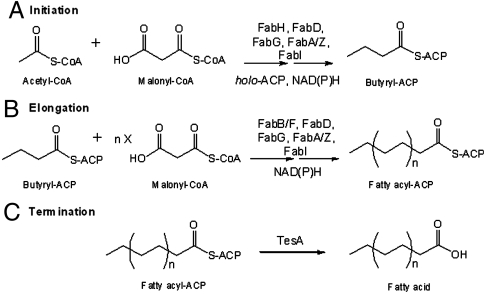
Fig. 1.
Catalytic cycle of the E. coli fatty acid synthase. (A) Initiation: In the presence of holo-ACP, NADPH and NADH, acetyl-CoA and malonyl-CoA undergo condensation and subsequent reduction to form butyryl-ACP. These reactions are catalyzed by the malonyl-CoA:ACP transacylase FabD, the ketosynthase FabH, the NADPH-dependent ketoreductase FabG, either the dual-function dehydratase/isomerase FabA or the monofunctional dehydratase FabZ, and the NADH-dependent enoyl reductase FabI. (B) Elongation: Butyryl-ACP is extended via 5–7 rounds of analogous reactions to produce a C14 to C18-ACP that is either fully saturated or monounsaturated. These extension cycles are catalyzed by either the ketosynthase FabB or FabF in collaboration with FabD, FabG, FabA or FabZ, and FabI. (C) Termination: The full-length fatty acid is released from the corresponding fatty acyl-ACP via hydrolysis by the thioesterase (TesA).