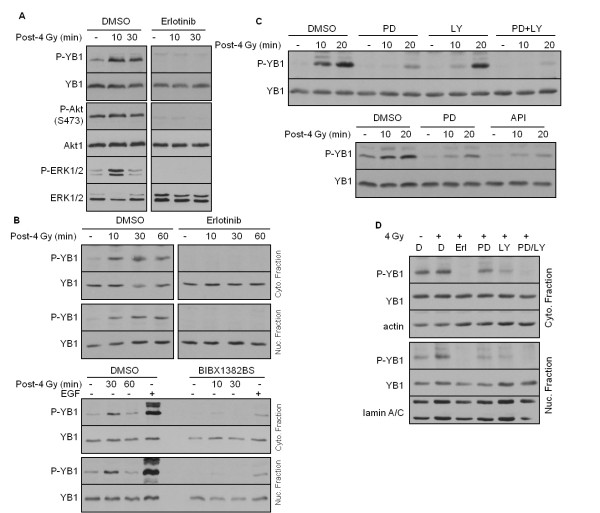
Figure 4.
Radiation-induced phosphorylation of YB-1 is mediated by erbB1-dependent PI3K/Akt and MAP kinase signaling. (A) SKBr3 cells were treated with dimethyl sulfoxide (DMSO) or erlotinib (5 μM) for 2 hours and mock-irradiated or irradiated with 4 Gy IR. At the indicated time points after irradiation, protein samples were isolated and P-YB-1, P-Akt and P-ERK1/2 were detected. The blots were stripped and incubated with antibodies against YB-1, ERK1/2 or Akt1. The effect of erlotinib on IR-induced YB-1 phosphorylation in whole cell extract was tested in two independent experiments. (B) SKBr3 cells were treated with DMSO or erlotinib and irradiated as described above. Thereafter 100 μg of isolated cytoplasmic and nuclear fractions were subjected to sodium dodecyl sulfate polyacrylamide electrophoresis. Blots from both fractions were incubated with P-YB-1, followed by stripping and detection of total YB-1. Actin in the cytoplasmic fraction was used as a loading control. The experiment was repeated using the most specific erbB1 tyrosine kinase inhibitor, BIBX1382BS. As a positive control, the 30-minute time point post-epidermal growth factor stimulation was included. (C and D) SKBr3 cells were treated with 20 μM PD98059 (PD), 10 μM LY294002 (LY), 2.5 μM API59CJ-OH (API), 5 μM erlotinib (Erl.) or a combination of PD98059 and LY294002 (PD/LY) for 2 hours. Control cells were treated with DMSO. Thereafter cells were irradiated with 4 Gy IR. (C) At the indicated time points and (D) 10 minutes after irradiation, protein samples were isolated and the levels of P-YB-1 were analyzed in (C) whole lysate and (D) cytoplasmic and nuclear fractions. Blots were stripped and reincubated with YB-1 antibody. Actin and lamin A/C were detected as loading controls. The experiments shown in Figures 4C and 4D were repeated at least twice, and representative Western blots are shown.