Figure 1.
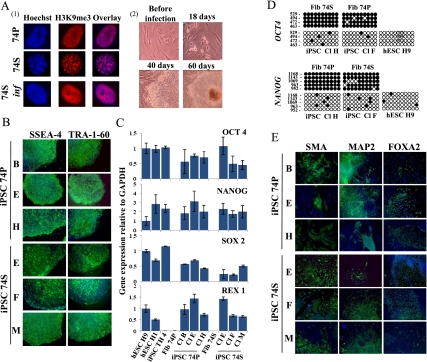
Induction of pluripotency in proliferative and senescent 74-yr-old-derived cells. (A, panel 1) Detection of SAHF by indirect immunofluorescence of H3K9me3 (red) and Hoechst (blue) in proliferative (74P), senescent (74S), and transduced senescent 74-yr-old cells (74S inf) by the six factors. Seven days after transduction, no SAHF were detected. (Panel 2) Eighteen days after transduction, proliferation of infected 74S cells was observed (74S inf). Around day 40, distinct colonies were observed. Representative phase-contrast images are shown. (B) Immunodectection of surface markers TRA-1-60 and SSEA-4 on iPSCs colonies derived from 74S- and 74P-year-old cells (three independent clones). (C) Quantitative RT–PCR of expression levels for endogenous pluripotency factors in the iPSCs from 74P and 74S and their parental fibroblasts. H1 and H9 hESCs and iPSC TH 4 were used as controls. Transcript levels were normalized to GAPDH expression. Error bars indicate standard deviations from duplicate experiments. (D) Bisulfite sequencing analysis of OCT4 and NANOG promoter regions showing demethylation in iPSCs from 74P and 74S, as in H9 hESCs, compared with parental fibroblasts. Each column of circles for a given amplicon represents the methylation status of CpG dinucleotides in one clone for that region. Open circles are unmethylated CpGs and closed circles methylated ones. The left numbers of each column indicate CpG localization relative to the transcriptional start site. (E) In vitro differentiation experiments of iPSCs reveal their potential to generate cell derivatives of all three primary germ cell layers. Immunodetection of SMA, MAP2, and FOXA2 markers specific for endoderm, ectoderm, and mesoderm, respectively. Nuclei are stained with Hoechst (blue). Three independent clones are shown.