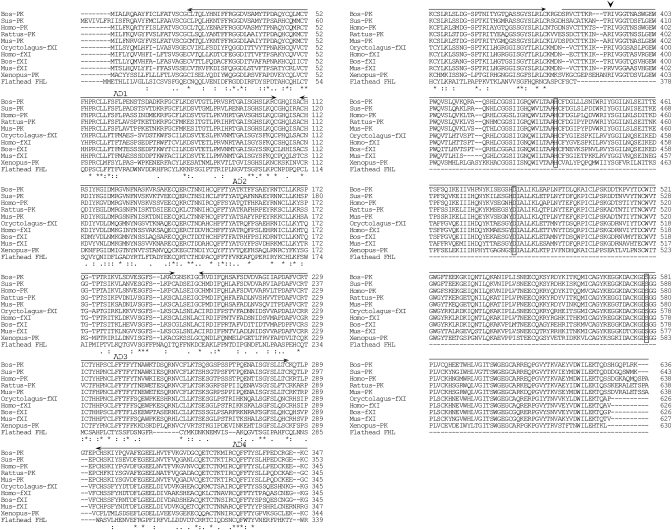
Fig. 3.
Comparison of the amino acid sequence of FHL with the plasma kallikreins and coagulation factor XIs of mammals and X. laevis. Numbers on the right indicate the amino acid position. Identical (*) and similar (: or .) residues identified by Clustal W are indicated. The cleavage site for enzyme activation in plasma kallikreins is indicated by arrowhead, and the catalytic triad residues (His, Asp and Ser) are boxed. Four apple domains (corresponding to human plasma kallikrein residues 21–104, 111–194, 201–284 and 292–375) are shown by arrows, and the upper line represents the serine protease domain. Abbreviations and accession numbers are as follows: Homo-PK, human H. sapiens plasma kallikrein (BC117351); Mus-PK, mouse M. musculus plasma kallikrein (BC026555); Rattus-PK, rat Rattus norvegicus plasma kallikrein (BC089815); Bos-PK, bovine Bos taurus plasma kallikrein (BC105498); Sus-PK, pig Sus scrofa plasma kallikrein (AB022425); Xenopus-PK, X. laevis plasma kallikrein (BC077417); Homo-fXI, H. sapiens factor XI (BC122863); Mus-fXI, M. musculus factor XI (BC019485); Bos-fXI, Bos taurus factor XI (AB196307) and Oryctolagus-fXI, rabbit Oryctolagus cuniculus factor XI (AF395821).