Abstract
OBJECTIVE
To estimate changes over time in birth weight for gestational age and in gestational length among term singleton neonates born from 1990 to 2005.
METHODS
We used data from the U.S. National Center for Health Statistics for 36,827,828 singleton neonates born at 37–41 weeks of gestation, 1990–2005. We examined trends in birth weight, birth weight for gestational age, large and small for gestational age, and gestational length in the overall population and in a low-risk subgroup defined by maternal age, race or ethnicity, education, marital status, smoking, gestational weight gain, delivery route, and obstetric care characteristics.
RESULTS
In 2005, compared with 1990, we observed decreases in birth weight (−52 g in the overall population, −79 g in a homogenous low-risk subgroup) and large for gestational age birth (−1.4% overall, −2.2% in the homogenous subgroup) that were steeper after 1999 and persisted in regression analyses adjusted for maternal and neonate characteristics, gestational length, cesarean delivery, and induction of labor. Decreases in mean gestational length (−0.34 weeks overall) were similar regardless of route of delivery or induction of labor.
CONCLUSION
Recent decreases in fetal growth among U.S., term, singleton neonates were not explained by trends in maternal and neonatal characteristics, changes in obstetric practices, or concurrent decreases in gestational length.
LEVEL OF EVIDENCE
III
In the United States, mean birth weight increased during the second half of the 20th century, continuing through the 1990s.1–4 Similar trends occurred in Canada,5–7 the United Kingdom,8–10 Scandinavia,11–13 and Japan.14 More recently, U.S. national surveillance data suggest that this upward trend in mean birth weight is reversing.15 However, those surveillance data include preterm births and multiple gestations and do not account for trends in maternal or neonatal characteristics or obstetric practices.
Birth weight is a composite of fetal growth and length of gestation, each of which has different contributors. Fetal growth (birth weight for gestational age) also increased from the 1980s to the 1990s among singleton births in the United States,2 and within a hospital-based cohort in Canada,7 but more recent data are scarce. The percentage of births that were preterm, ie, that occurred before 37 completed weeks of gestation, has also increased since 1990, including an increase in late preterm deliveries.15,16 The extent to which gestation duration has changed among term births has not been well studied.
The purpose of the present analysis was to estimate trends in birth weight for gestational age in the United States among singleton term births from 1990 through 2005. We hypothesized that, after accounting for declines in gestational length, birth weight would have continued to increase during this period, with resulting increases in the percentage of large for gestational age births, and that trends in maternal characteristics, especially gestational weight gain, would explain these increases.
MATERIALS AND METHODS
We used data from the National Center for Health Statistics Natality Data Sets for the years 1990–2005.15 This study was reviewed by the Human Subjects Committee of Harvard Pilgrim Health Care and found to be exempt under paragraph #4 of the Department of Health and Human Services Code of Regulations.
We limited our analyses to singletons born at 37 to 41 completed weeks of gestational age to U.S. resident mothers aged 18 years and older. We excluded births in California, which does not report maternal weight gain during pregnancy. We excluded births in which gestational age was imputed or plurality, birth weight, or neonatal sex was missing. We next trimmed the data to exclude birth weights inconsistent with gestational age, based on the method of Alexander et al.17 For births at 37 completed weeks of gestation, we excluded birth weights less than 1,000 g or greater than 5,500 g, and for births at 38 completed weeks or greater, we excluded birth weights 1,000 g or greater than 6,000 g. We thus retained information on 36,827,828 babies and their mothers, ranging from a low of 2,160,396 births in 1991 to a high of 2,579,198 births in 2003.
Birth weight was recorded in grams. Gestational age, calculated from the date of the last menstrual period, was reported in completed weeks. We defined small for gestational age (SGA) as fetal growth less than 10th percentile and large for gestational age (LGA) as greater than 90th percentile at each completed week of gestation, according to published reference data based on births that occurred 1999–2000.18 We compared all measures to baseline values in 1990. We also obtained information from the U.S. Natality dataset on maternal and obstetric factors. For all analyses we categorized maternal characteristics as follows: maternal age (18–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54 years), race/ethnicity (Mexican, Puerto Rican, Cuban, Central or South American, other Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic other race), education (0 – 8, 9 – 11, 12, 13 – 15, 16 years and more), tobacco use (nonsmoker, used tobacco during pregnancy), marital status (married, not married), timing of prenatal care initiation (first, second, or third trimester; no prenatal care; unknown), and maternal gestational weight gain (less than 16, 16 to less than 21, 21 to less than 26, 26 to less than 31, 31 to less than 35, 36 to less than 41, 41 to less than 46, 46 pounds or greater). We obtained information on nine obstetric procedures and medical risk factors: delivery route (primary or repeat cesarean delivery, vaginal delivery), induction of labor, prenatal ultrasonography, chronic hypertension, gestational hypertension, eclampsia, previous preterm birth, and previous newborn 4,000 g or greater. One category indicated both pregestational and gestational diabetes. We also obtained information on neonatal sex and birth order.
A standard U.S. certificate of birth has been in use since 1989, but in 2003 a revised birth certificate entered use, with gradual adoption on a state-by-state basis. The percentage of births in the analytic dataset that were recorded on the 2003 birth certificate increased from 6% in 2003 to 35% in 2005. We set to “missing” maternal smoking during pregnancy and previous preterm birth recorded on the 2003 version of the birth certificate, as these variables were not comparable with the 1989 version. We recoded timing of initiation of prenatal care and maternal education so that they were comparable with the 1989 birth certificate categories. Other variables, including birth weight and gestational age at birth, did not differ between birth certificate versions.
To minimize the influence of measured maternal and obstetric factors, we also repeated our analyses among 502,716 (1.4%) low-risk women, whom we defined as age 25–29 years, non-Hispanic white race/ethnicity, greater than 12 years of education, married, received prenatal care in first trimester, nonsmoker, no medical complications during current or previous pregnancy, delivered vaginally, labor not induced, had an ultrasound examination, and gained 26–35 pounds during the current pregnancy.
We also performed multivariable analyses using linear and logistic regression models to account for maternal and neonatal characteristics and obstetric practices. In separate models for each birth outcome, we examined associations of year of birth with birth weight and gestational age at birth. We first performed unadjusted analyses and then we sequentially adjusted for maternal and neonatal characteristics. Maternal prepregnancy body mass index (BMI) was not included on the 1989 version of the birth certificate. To account for secular trends in maternal BMI and height, we assigned each woman the mean BMI and height among nonpregnant women aged 18–54 years recorded in corresponding years in the National Health and Examination Survey,19 and included these variables in regression models. Given the large sample size, all comparisons were associated with very small P values (<.001) and narrow confidence intervals; therefore, we present only effect estimates. We performed all analyses using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
In Table 1 we present information on selected maternal and obstetric characteristics for the years 1990, 1995, 2000, and 2005. During this period no marked temporal trends were apparent in neonatal sex (51.1% boys in 1990 and 51.0% in 2005), the percentage of births to nulliparous women (30.1% and 31.6%), or the percentage of women who received prenatal care in the first trimester (79.9% and 80.3%). However, an increasing percentage of neonates were born to mothers with characteristics associated with higher fetal growth, namely age greater than 35 years, education 16 years or greater, diabetes before or during pregnancy, gestational weight gain 46 pounds or greater, or nonsmoker during pregnancy (Table 1). More neonates were also born to mothers with other characteristics associated with lower fetal growth, namely hypertension with onset before or during pregnancy, gestational weight gain less than 16 pounds, Hispanic or non-Hispanic black race/ethnicity, or unmarried (Table 1). Trends in these maternal characteristics were fairly constant across time, and none changed direction during the 16-year observational period. In contrast, the percentage of births delivered by cesarean delivery decreased from 22.0% in 1990 to 19.8% in 1995 and thereafter increased to 28.3% in 2005. Induction of labor and the use of prenatal ultra-sonography each increased over time.
Table 1.
Mean Birth Weight Among Neonates Born at 37–41 Weeks of Gestation and Proportion of Births at 40–41 Weeks of Gestational Age, by Maternal Characteristics and Obstetric Procedures Among Singleton Births in the United States, 1990–2005
| Characteristic | 1990
|
1995
|
2000
|
2005
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Mean (SD) Birth Weight (g) | 40–41 Weeks of Gestation (%) | % | Mean (SD) Birth Weight (g) | 40–41 Weeks of Gestation (%) | % | Mean (SD) Birth Weight (g) | 40–41 Weeks of Gestation (%) | % | Mean (SD) Birth Weight (g) | 40–41 Weeks of Gestation (%) | |
| Overall | 3,441 (486) | 47.4 | 3,435 (483) | 44.5 | 3,429 (478) | 40.0 | 3,389 (466) | 34.1 | ||||
| Age greater than 35 | 9.4 | 3,497 (508) | 42.7 | 12.2 | 3,483 (502) | 39.9 | 13.8 | 3,485 (496) | 35.7 | 14.3 | 3,448 (480) | 29.3 |
| Hispanic | 9.7 | 3,391 (466) | 44.4 | 12.0 | 3,391 (464) | 42.9 | 15.5 | 3,396 (464) | 40.2 | 19.5 | 3,369 (455) | 35.0 |
| Non-Hispanic black | 14.1 | 3,265 (482) | 40.4 | 13.9 | 3,271 (480) | 39.7 | 14.1 | 3,277 (475) | 37.2 | 13.6 | 3,244 (465) | 31.7 |
| Education 16 years or greater | 21.1 | 3,517 (467) | 48.1 | 25.2 | 3,508 (466) | 45.0 | 28.0 | 3,499 (463) | 40.4 | 29.4 | 3,456 (453) | 34.3 |
| Unmarried | 21.1 | 3,305 (488) | 45.8 | 26.2 | 3,19 (483) | 44.7 | 28.4 | 3,327 (478) | 41.4 | 33.0 | 3,307 (468) | 35.9 |
| Smoked during pregnancy | 17.0 | 3,257 (480) | 46.3 | 12.9 | 3,244 (481) | 43.3 | 11.1 | 3,249 (476) | 39.5 | 10.2 | 3,223 (465) | 33.3 |
| Diabetes* | 2.4 | 3,545 (543) | 36.3 | 2.7 | 3,552 (546) | 32.5 | 3.0 | 3,517 (544) | 27.5 | 3.9 | 3,459 (524) | 22.7 |
| Hypertension* | 3.2 | 3,371 (561) | 38.4 | 3.9 | 3,341 (553) | 33.3 | 4.3 | 3,329 (545) | 28.5 | 4.6 | 3,291 (528) | 23.4 |
| Gestational weight gain less than 16 lb | 8.0 | 3,286 (502) | 42.8 | 9.7 | 3,308 (495) | 40.8 | 10.7 | 3,314 (488) | 36.8 | 12.0 | 3,278 (471) | 30.9 |
| Gestational weight gain 46 lb or greater | 9.1 | 3,641 (500) | 53.1 | 10.2 | 3,612 (496) | 49.5 | 11.8 | 3,586 (487) | 44.0 | 12.9 | 3,538 (477) | 37.6 |
| Cesarean delivery | 22.0 | 3,486 (530) | 42.1 | 19.8 | 3,490 (538) | 40.2 | 21.3 | 3,479 (530) | 35.2 | 28.3 | 3,423 (507) | 28.7 |
| Induction of labor | 10.3 | 3,499 (514) | 55.4 | 17.7 | 3,491 (500) | 51.1 | 22.3 | 3,470 (487) | 44.8 | 25.6 | 3,419 (466) | 39.1 |
| Ultrasonography | 56.6 | 3,446 (490) | 47.1 | 64.2 | 3,438 (488) | 44.3 | 69.7 | 3,434 (479) | 40.0 | 72.4 | 3,393 (466) | 34.2 |
SD, standard deviation.
Prepregnancy or gestational.
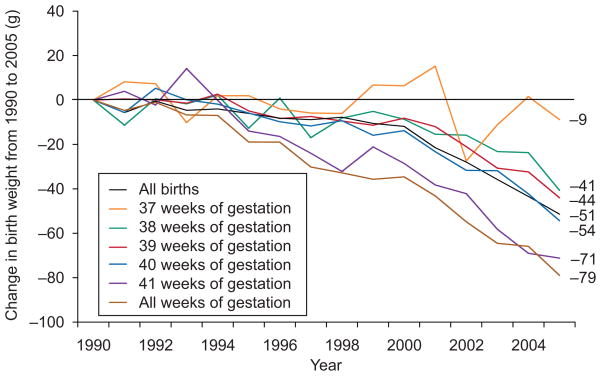
Mean birth weight among all term singleton neonates decreased by 52 g during the 16-year study period (Table 1 and Fig. 1). Mean birth weight differed by maternal characteristics and obstetric practices, but mean birth weight decreased from 2000 to 2005 within every maternal characteristic (Table 1). Declines over time were greater in the homogenous low-risk subgroup (−79 g from 1990 to 2005), in which mean birth weight declined within each completed week of gestational age (Fig. 1). Decreases were least steep among neonates born at 37 weeks (−9 g) and greatest among those born at 41 weeks (−71 g).
Fig. 1.
Trends in birth weight from 1990 to 2005 among all 36,827,828 singleton births born at 37 to 41 completed weeks of gestation and by gestational age at birth among a low-risk subgroup of 502,716 singleton neonates born to mothers of non-Hispanic white race/ethnicity with 13 or more years of education and of married status, who received prenatal care in the first trimester, were non-smokers, had no pregnancy complications, delivered vaginally, did not have labor induced, had a prenatal ultrasound examination, and gained 26 to 35 pounds during pregnancy.
Donahue. Trends in Birth Weight in the United States. Obstet Gynecol 2010.
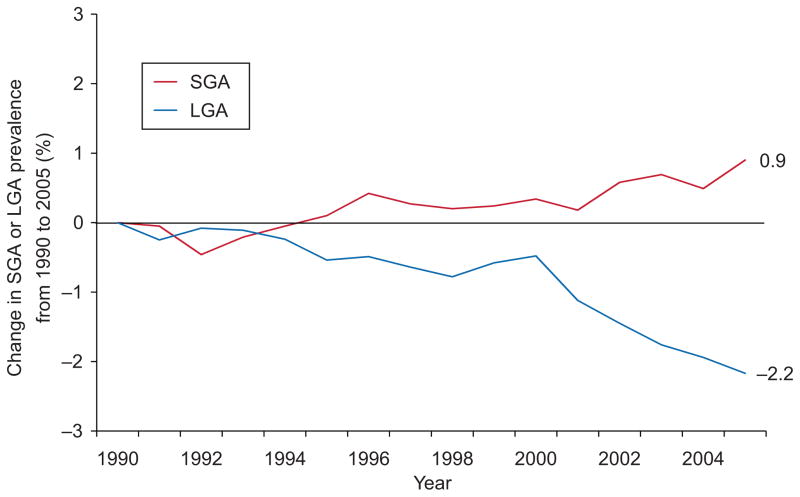
In the overall population, rates of LGA remained fairly stable at about 10.3% until 2000 and thereafter steadily dropped to 8.9% in 2005. Decline in LGA prevalence was even greater in the homogenous low-risk subgroup (−2.2%, from 9.4% to 7.2%, Fig. 2) and was apparent at every gestational age (1.7% lower in 2005 compared with 1990 among neonates born at 37 weeks of gestation, 3.3% lower among those born at 41 weeks of gestation). In the overall population, the percentage of neonates born SGA remained steady from 1990 to 1996 at about 10.3%, declined slightly until 1999, and thereafter increased back to 10.2% in 2005. In the low-risk subgroup, however, the prevalence of SGA steadily increased from 1992 to 1996 and again from 2001 to 2005 and was 0.9% higher in 2005 (8.1%) than in 1990 (7.2%, Fig. 2). The proportion of births that were SGA was about 1% higher in 2005 compared with 1990 for all weeks of gestation except for births at 37 weeks of gestation (−0.1% change), although in this group numbers were small and therefore estimates were somewhat unstable.
Fig. 2.
Trends in prevalence of small-for-gestational age (SGA) and large-for-gestational age (LGA) births from 1990 to 2005 among 502,716 singletons born at 37 to 41 completed weeks of gestation to low-risk mothers aged 25 to 29 years of non-Hispanic white race/ethnicity, 13 or more years of education, and of married status, who received prenatal care in the first trimester, were non-smokers, had no pregnancy complications, delivered vaginally, did not have labor induced, had a prenatal ultrasound examination, and gained 26 to 35 pounds during pregnancy.
Donahue. Trends in Birth Weight in the United States. Obstet Gynecol 2010.
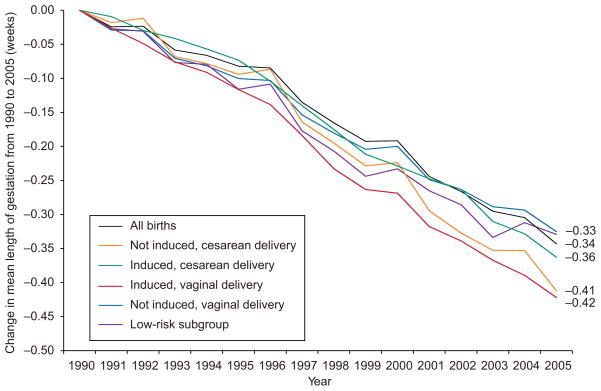
From 1990 to 2005 the gestational length distribution in this population of term births shifted toward shorter gestation duration, with a mean decrease of 0.34 weeks (2.4 days) in the overall population and 0.33 weeks in the low-risk subgroup (Fig. 3). Although mean gestational length differed by obstetric management characteristics, declines from 1990 to 2005 were similar for neonates born after induction of labor (−0.42 weeks), by cesarean delivery (−0.41 weeks), or both (−0.36 weeks, Fig. 3). The proportion of births at 40–41 weeks of gestation declined in all subgroups in parallel with decreases in mean gestational length (data not shown).
Fig. 3.
Trends in length of gestation from 1990 to 2005, according to induction of labor status and route of delivery, among 36,827,828 singletons born at 37 to 41 completed weeks of gestation, and among a low-risk subgroup of 502,716 singleton neonates born to mothers aged 25 to 29 years, of non-Hispanic white race/ethnicity, with 13 or more years of education, of married status, who received prenatal care in the first trimester, were nonsmokers, had no pregnancy complications, delivered vaginally, did not have labor induced, had a prenatal ultrasound examination, and gained 26 to 35 pounds during pregnancy.
Donahue. Trends in Birth Weight in the United States. Obstet Gynecol 2010.
Restricting analyses to a low-risk subgroup is one way to account for trends in maternal and neonatal characteristics and obstetric practices. We also used multivariable regression analyses. Results of fully adjusted regression analyses were similar to results within the homogenous low-risk subgroup. For example, in an unadjusted model using year of birth as a linear predictor, birth weight declined by 3.0 g/year, with a steeper decline after serial adjustment for maternal and neonatal characteristics (−3.7 g/year) and obstetric practices (−4.2 g/year). This decline over time was attenuated but not eliminated by additional adjustment for gestational age at birth (−1.9 g/year) but then strengthened after adjustment for secular trends in maternal prepregnancy BMI and height (−3.6 g/year). The odds of birth at 40 – 41 compared with 37–39 weeks declined over time and was not substantially different after adjustment for maternal and neonatal characteristics and obstetric management practices (unadjusted odds ratio 0.58, fully adjusted odds ratio 0.57 for 2005 compared with 1990).
DISCUSSION
From 1990 to 2005, birth weight decreased among term births in the United States, especially after 1999. These declines were not explained by statistical adjustment for maternal and neonatal characteristics or obstetric practices, such as cesarean delivery, induction of labor, and use of prenatal ultrasonography, and were similar in analyses restricted to a homogenous subset of low-risk mothers. Large for gestational age birth decreased, whereas small for gestational age birth increased.
Strengths of this study include the large nationally representative sample. We had information on a large number of maternal and obstetric characteristics associated with fetal growth and gestational age. Limitations are inherent to birth record data. Birth weight tends to be well recorded, but other factors including gestational length may not be as accurate. The Natality datasets do not include an estimate of gestational length based on ultrasound dating. However, gestational age calculated by the date of the last menstrual period tends to be most accurate among term births.20 The percentage of missing data varied over time for some variables, most notably gestational weight gain (12% missing in 1990 compared with 4% in 2005). However, declines in birth weight were even stronger among the subset of low-risk mothers with no missing data. It is possible that maternal characteristics were not well recorded. It is unlikely that the introduction of the 2003 version of the birth certificate would explain our results, as most factors, including birth weight and gestational age at birth, were recorded identically on the two forms, and downward trends in fetal growth preceded adoption of the revised certificate. Information on augmentation of labor was not available on the 1989 birth certificate, and in some cases induction of labor might be miscoded as augmentation, or vice versa. Maternal prepregnancy BMI, height, and use of assisted reproductive technologies were also not available. As these variables are included on the 2003 birth certificate revision,21 future studies might be better able to track their associations with birth outcomes.
Previous studies, most including only births that occurred before 2000, have evaluated trends in fetal growth and its contributors. In a hospital-based study in Canada using data from 1978 to 1996, trends in maternal characteristics such as increasing prepregnancy BMI and gestational weight gain and decreasing prevalence of smoking during pregnancy explained the observed increases in birth weight and fetal growth.7 Similarly, among neonates born in Sweden from 1992 to 2001, a 23% increase in LGA among term singleton births was explained by concurrent increases in maternal BMI and decreases in smoking.12 Increases in births greater than 4,000 g in Denmark from 1990 to 1999 were explained by changes in maternal prepregnancy weight, height, smoking habits, educational level, and caffeine intake.22 In an analysis of term neonates from 1985 to 1998 using U.S. and Canadian birth data, increases in birth weight and LGA, and decreases in SGA, were attributed to preterm obstetric induction and preterm cesarean delivery.2 A recent study from Sweden reported that, after adjustment for gestational length, age, smoking, parity, and employment, birth weight increased within all maternal BMI categories from 1978 to 1992, but thereafter decreased among neonates born to normal-weight women.23
In the present study, the maternal characteristics routinely recorded on the birth certificate did not appear to be responsible for observed decreases in fetal growth. This observation is concordant with the fact that the directions of trends in all maternal characteristics have continued since the early 1990s without any reversals. Since these trends explained past increases in birth weight and fetal growth, they could not explain recent decreases. Induction of labor also steadily increased each year after 1990. Although rates of cesarean deliveries declined in the early 1990s and subsequently increased after 1997, declines in fetal growth preceded 1997 among some subgroups and were similar in neonates delivered by cesarean and vaginal routes. Thus, it is unlikely that shifts in cesarean delivery practices account for observed trends in fetal growth.
Gestational age is a strong contributor to birth weight. From 1990 to 2005, mean gestational length among U.S. term births decreased by more than 2 days, and the odds of birth on or after the “due date” decreased by more than 40%. Decreases in gestation duration occurred among neonates born by vaginal delivery or by cesarean delivery and among those whose labor was or was not induced. Neither statistical adjustment for maternal and neonatal characteristics and obstetric practices nor restriction of the sample to a homogenous low-risk subgroup–none of whom were induced or delivered by cesarean section–attenuated observed declines in gestational duration among these term births. Similarly, among all subgroups, the proportion of births at 40–41 weeks declined in parallel with mean gestational length (data not shown).
However, the observed decreases in gestational length did not entirely explain the declines in birth weight, fetal growth, and LGA birth, which were somewhat less strong but still persisted after statistical adjustment for gestational age at birth and within specified weeks of gestation. Gestational age at birth is reported on birth certificates in completed weeks and not more finely. It is possible that a decrease of gestational length of a few days within each gestational week might account for the observed declines in fetal growth. If, for example, babies were less likely over time to be born at 39 6/7 weeks and more likely to be born at 39 1/7 weeks, there would be an apparent decline in birth weight at 39 weeks that in fact resulted from a decrease in gestational length rather than a true decrease in fetal growth. However, if such differences in gestational length occurred universally (eg, other neonates who would have been born at 40 1/7 weeks were now born at 39 6/7 weeks), we would observe no such decline in birth weight.
Change over time in the assessment of gestational age may affect observations of trends in gestational length and birth weight for gestational age, although less strongly among term than preterm neonates.24 If smaller neonates were over time increasingly likely to be classified as term rather than preterm, then we would see a downward trend in birth weight for gestational age, especially in gestational weeks 37–38. However, the decline in birth weight was in fact most pronounced in neonates born at 40–41 weeks of gestation. Alternatively, if gestational age at delivery was increasingly over time reclassified lower, that might explain the trends that we observed, as neonates born at 42 weeks or greater tend to be smaller than those born at 40–41 weeks, who are in turn larger than those born at 37–38 weeks.18 However, we also saw decreasing size at birth among the low-risk subgroup, all of whom had first-trimester prenatal care and a prenatal ultrasonography, and presumably equally accurate determination of gestational age over time.
It is also possible that neonates with faster intra-uterine growth were over time increasingly more likely to be delivered earlier because of greater use of cesarean delivery or induction of labor for these larger neonates. In this case, we would expect to see declines in birth weight that were greatest in the later gestational ages, as we did observe. In the regression analysis, however, we did not see evidence that adjustment for obstetric management practices, namely prenatal ultrasonography, induction of labor, and cesarean delivery, attenuated the estimates of decreasing fetal growth over time, but rather seemed to strengthen these estimates. Similarly, birth weight declined even among the homogenous, low-risk subset of mothers, and among all mothers who had neither cesarean delivery nor induction of labor. Classification of cesarean delivery on birth certificates is quite accurate, with 99.8% sensitivity.25 However, induction of labor may not be as accurately recorded, and thus some births that were in fact induced may have been misclassified.
Maternal prepregnancy BMI, a strong contributor to fetal growth,7 was not included on the 1989 birth certificate version. However, since BMI among women of childbearing age has increased over time,26 we would expect that accounting for increasing prepregnancy BMI trends should adjust fetal growth estimates further downward, as we observed with our relatively crude adjustment using mean BMI obtained from the National Health and Examination Survey.
Other factors not recorded in birth records that might contribute to declines in gestational length or fetal growth include trends in maternal diet, physical activity, stress, socioeconomic factors, pollution or toxicant exposures, or prevalence of other, unrecorded medical conditions such as asthma. More detailed studies of smaller populations would be needed to explore the role of these factors.
In this study of term singleton births in the United States from 1990 to 2005, we observed decreases in birth weight that were not explained by maternal and neonatal characteristics or by trends in induced labor or cesarean delivery. Parallel declines in gestational age at birth did not entirely explain the decreasing birth weight. Size at birth predicts not only short-term complications but also long-term health and chronic disease risk even among term births.27 Although the consequences of the modest differences over time in birth weight for gestational age that we observed here are uncertain, any underlying reasons for such a decline may themselves directly influence child health. Therefore, active investigation into the determinants and longer-term sequelae of declining fetal growth among term births is warranted.
Acknowledgments
Supported by grants from the National Institutes of Health (HD 44807, HL 68041).
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Institute of Medicine. Nutrition during pregnancy. Washington, DC: National Academy Press; 1990. [Google Scholar]
- 2.Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Semin Perinatol. 2002;26:260–7. doi: 10.1053/sper.2002.34772. [DOI] [PubMed] [Google Scholar]
- 3.Wen SW, Kramer MS, Platt R, Demissie K, Joseph KS, Liu S, et al. Secular trends of fetal growth in Canada, 1981 to 1997. Paediatr Perinat Epidemiol. 2003;17:347–54. doi: 10.1046/j.1365-3016.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- 4.Chike-Obi U, David RJ, Coutinho R, Wu SY. Birth weight has increased over a generation. Am J Epidemiol. 1996;144:563–9. doi: 10.1093/oxfordjournals.aje.a008966. [DOI] [PubMed] [Google Scholar]
- 5.Arbuckle TE, Sherman GJ. An analysis of birth weight by gestational age in Canada. CMAJ. 1989;140:157–60. 165. [PMC free article] [PubMed] [Google Scholar]
- 6.Millar WJ, Strachan J, Wadhera S. Trends in low birthweight Canada. 1971 to 1989. Health Rep. 1991;3:311–25. [PubMed] [Google Scholar]
- 7.Kramer MS, Morin I, Yang H, Platt RW, Usher R, McNamara H, et al. Why are babies getting bigger? Temporal trends in fetal growth and its determinants. J Pediatr. 2002;141:538–42. doi: 10.1067/mpd.2002.128029. [DOI] [PubMed] [Google Scholar]
- 8.Alberman E. Are our babies becoming bigger? J R Soc Med. 1991;84:257–60. doi: 10.1177/014107689108400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power C. National trends in birth weight: implications for future adult disease. BMJ. 1994;308:1270–1. doi: 10.1136/bmj.308.6939.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonellie SR, Raab GM. Why are babies getting heavier? Comparison of Scottish births from 1980 to 1992. BMJ. 1997;315:1205. doi: 10.1136/bmj.315.7117.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79:440–9. [PubMed] [Google Scholar]
- 12.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104:720–6. doi: 10.1097/01.AOG.0000141442.59573.cd. [DOI] [PubMed] [Google Scholar]
- 13.Orskou J, Kesmodel U, Henriksen TB, Secher NJ. An increasing proportion of infants weigh more than 4000 grams at birth. Acta Obstet Gynecol Scand. 2001;80:931–6. doi: 10.1034/j.1600-0412.2001.801010.x. [DOI] [PubMed] [Google Scholar]
- 14.Oishi K, Honda S, Takamura N, Kusano Y, Abe Y, Moji K, et al. Secular trends of sizes at birth in Japanese healthy infants born between 1962 and 1988. J Physiol Anthropol Appl Human Sci. 2004;23:155–61. doi: 10.2114/jpa.23.155. [DOI] [PubMed] [Google Scholar]
- 15.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: Final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- 16.Engle WA, Tomashek KM, Wallman C. Committee on Fetus and Newborn, American Academy of Pedatrics “Late-pre-term” infants: a population at risk. Pediatrics. 2007;120:1390–401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 17.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 18.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anonymous. [Retrieved December 10, 2009];National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/nhanes.htm.
- 20.Kramer MS, McLean FH, Boyd ME, Usher RH. The validity of gestational age estimation by menstrual dating in term, preterm, and postterm gestations. JAMA. 1988;260:3306–8. [PubMed] [Google Scholar]
- 21.Martin JA, Menacker F. Expanded health data from the new birth certificate, 2004. Natl Vital Stat Rep. 2007;55:1–22. [PubMed] [Google Scholar]
- 22.Orskou J, Henriksen TB, Kesmodel U, Secher NJ. Maternal characteristics and lifestyle factors and the risk of delivering high birth weight infants. Obstet Gynecol. 2003;102:115–20. doi: 10.1016/s0029-7844(03)00402-2. [DOI] [PubMed] [Google Scholar]
- 23.Brynhildsen J, Sydsjo A, Ekholm-Selling K, Josefsson A. The importance of maternal BMI on infant’s birth weight in four BMI groups for the period 1978–2001. Acta Obstet Gynecol Scand. 2009;88:391–6. doi: 10.1080/00016340902807199. [DOI] [PubMed] [Google Scholar]
- 24.Sinclair JC, Blidner IN. An analysis of birth weight by gestational age in Canada. CMAJ. 1989;141:375–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Green DC, Moore JM, Adams MM, Berg CJ, Wilcox LS, McCarthy BJ. Are we underestimating rates of vaginal birth after previous cesarean birth? The validity of delivery methods from birth certificates. Am J Epidemiol. 1998;147:581–6. doi: 10.1093/oxfordjournals.aje.a009490. [DOI] [PubMed] [Google Scholar]
- 26.Yeh J, Shelton JA. Increasing prepregnancy body mass index: analysis of trends and contributing variables. Am J Obstet Gynecol. 2005;193:1994–8. doi: 10.1016/j.ajog.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]