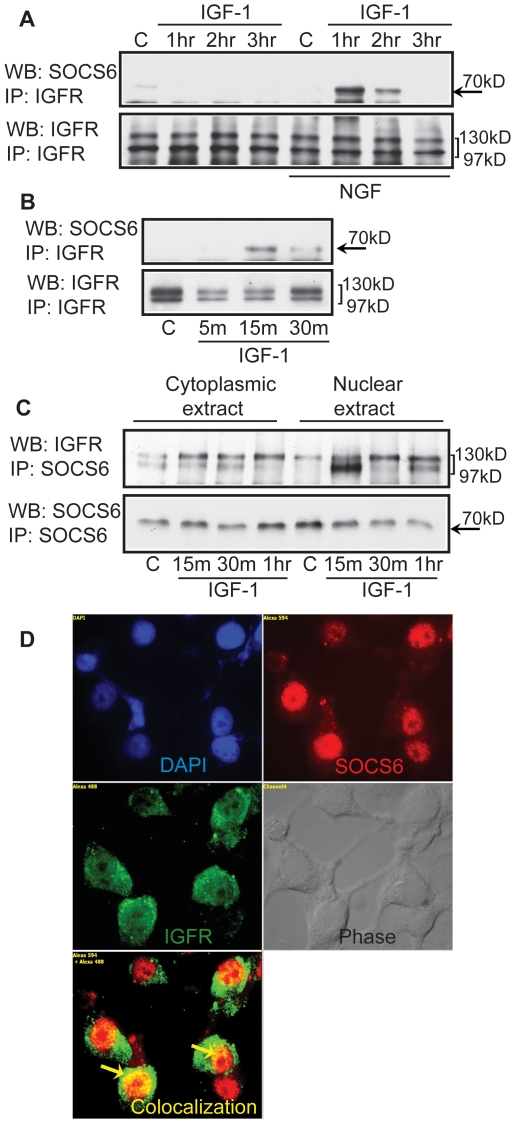
Figure 9. SOCS6 associates with IGFR upon IGF-1 stimulation.
(A) Undifferentiated or NGF differentiated PC12 cells were stimulated with/without IGF-1 for 1, 2 or 3 hours. Using 300 µg of cell lysate, IGFR was pulled down and Western-blotted with anti-SOCS6 antibody. The membrane was stripped and reprobed with anti-IGFR antibody for loading control. (B) SOCS6 stable PC12 cells were stimulated with/without IGF-1 for 5, 15, and 30 minutes. IGFR was immunoprecipitated and Western-blotted with anti-SOCS6 antibody. The membrane was stripped and reprobed with anti-IGFR antibody for loading control. (C) PC12 cells were differentiated for 2 days with NGF and then stimulated with/without IGF-1 for 15, 30 m or 1 hr (m = minutes and hr = hours) and nuclear and cytoplasmic protein was extracted (as described in experimental procedures). Using 300 µg of cell lysate, SOCS6 was pulled down and Western-blotted with anti-IGFR antibody. The membrane was stripped and reprobed with anti-SOCS6 antibody for loading control. (D) PC12 cells were allowed to differentiate for 2 days with NGF and stimulated with IGF-1 for 30 minutes. The cells were then fixed and permeabilized. After primary antibody treatment (anti-SOCS6, anti-IGFR and anti-SOCS6+anti-IGFR), SOCS6 was stained with Alexafluor 594 (red) and IGFR was stained with Alexafluor 488 (green). The cells were visualized under fluorescent microscope. DAPI staining shows the location of the nucleus.