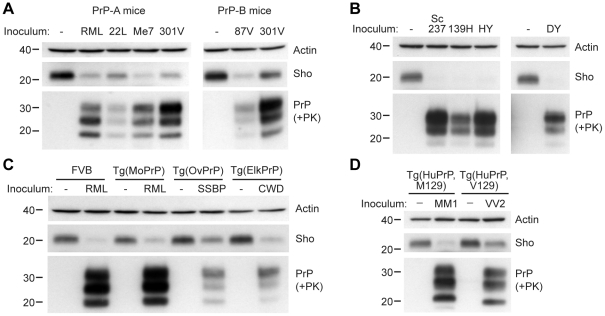
Figure 3. Decreased Sho levels in different animal models infected with diverse prion strains.
(A) Western blot analysis of brain homogenates prepared from wt FVB mice (expressing the PrP-A allotype) infected with RML, 22L, Me7, and 301V prions, and B6.I mice (expressing the PrP-B allotype) infected with 87V and 301V prions. All inoculated mice developed prion disease, as indicated by the presence of protease-resistant PrPSc, and showed decreased Sho levels. (B) Western blot analysis of brain homogenates from hamsters infected with Sc237, 139H, HY and DY prion strains. All inoculated hamsters developed prion disease, as indicated by the presence of protease-resistant PrPSc, and showed depleted Sho levels. (C) In Tg(OvPrP) infected with scrapie SSBP prions and Tg(ElkPrP) mice infected with elk CWD prions, Sho levels were decreased compared to age-matched, uninfected animals. In addition to decreased Sho levels, clinically ill animals showed protease-resistant PrPSc in their brains. Wild-type mice and Tg mice overexpressing mouse PrP are shown for comparison. (D) Western blot analysis of brain homogenates prepared from Tg(HuPrP,M129) and Tg(HuPrP,V129) mice infected with human sCJD(MM1) and sCJD(VV2) prions, respectively. Clinically ill mice showed reduced levels of Sho compared to uninfected controls. For all panels, actin levels are shown as a control. Sho was probed with the antibody 06rSH-1; PrP was detected using antibodies HuM-D18 (A); 3F4 (B, D); or HuM-P (C), respectively. Molecular masses based on the migration of protein standards are shown in kilodaltons.