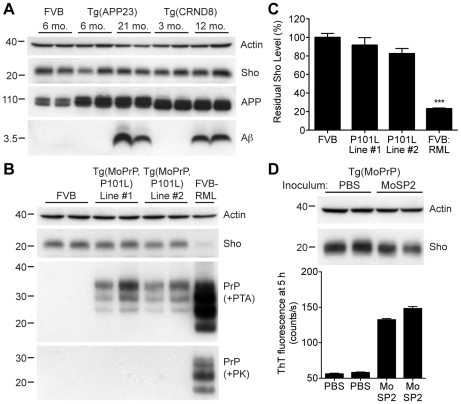
Figure 5. Unchanged Sho levels in mice with other neurodegenerative illnesses.
(A) Levels of Sho in the brains of Tg(APP23) and Tg(CRND8) mice, two Tg mouse models of Alzheimer's disease, were unaltered despite the high levels of cerebral Aβ present in aged mice. Actin and amyloid precursor protein (APP) levels are shown as controls. For comparison, Sho, APP, and Aβ levels in the brain of a wt FVB mouse are shown. Sho was probed with the antibody 06rSH-1; Aβ detected with the antibody 6E10, and APP with the antibody APPCT. (B) No change in Sho levels in either of two lines of Tg mice with a neurodegenerative disease caused by expression of mutant MoPrP(P101L). The brains of these mice have abundant levels of protease-sensitive, PTA-precipitable PrP but do not have any PK-resistant PrP. Sho and protease-resistant PrPSc in wt FVB mice infected with RML prions are shown for comparison. Sho was detected with the antibody 06rSH-1 and PrP was probed with the antibody HuM-D18. Actin levels are shown as a control. (C) Quantification of Sho levels in Tg(MoPrP,P101L) mice reveal only small decreases compared to wt mice (n = 3 for each group). In contrast, Sho levels in wt mice infected with RML prions are decreased by ∼80% (***P<0.001) compared to uninfected, wt mice. (D) Levels of Sho in the brains of Tg4053 mice overexpressing MoPrP inoculated with the MoSP2 strain of protease-sensitive prions were similar to those in age-matched Tg4053 mice inoculated with PBS. Actin levels are shown as a control. The presence of protease-sensitive prions in the brains of MoSP2-infected Tg4053 mice was confirmed by their ability to seed the polymerization of recombinant PrP into amyloid as demonstrated by increased ThT fluorescence signals in RT-QuIC experiments. Sho was detected with the antibody 06rSH-1. For all Western blots, molecular masses based on the migration of protein standards are shown in kilodaltons.