Abstract
To produce better antibacterial water-insoluble nanocomposites of silver (Ag), silver–silicon dioxide (Ag-SiO2) hybrid and silver colloid (Ag-c) nanoparticles (NPs) were studied. Ag-c NPs were synthesized using reduction of AgNO3, and Ag-SiO2 composites were prepared on a core of silica NPs functionalized with ethylenediamino-propyltrimethoxysilane, where Ag clusters were fabricated on amino groups using seed-mediated growth and characterized by transmission electron microscopy and ultraviolet-visible absorption spectroscopy. Antibacterial, effectiveness of the Ag-SiO2 NPs was tested against general Escherichia coli (E. coli ATCC 25922) and E. coli O157:H7 by measuring the growth based on optical density and digital counting of live-dead cells using a fluorescent microscope, and a field emission scanning electron microscope. Minimum inhibitory concentration values were studied against four representative bacteria along with E. coli O157:H7. Results showed that Ag NPs of 6.6 ± 4.5 nm were attached to the surface of SiO2 NPs (74 ± 13.5 nm), and the Ag-c NPs (3.5 ± 2 nm) showed excellent antibacterial properties.
Keywords: Ag-SiO2 nanoparticles, Ag colloids, Transmission electron microscopy, Field emission scanning electron microscopy, Optical density, Antibacterial
The antibacterial property of silver (Ag) has been known for thousands of years, and the ancient Greeks cooked in silver pots. The adage, “born with a silver spoon in his mouth” referred to more than simply wealth, and it was well known that eating with a silver spoon was more hygienic.1 Currently, the investigation of this phenomenon has gained importance as a result of the increase in bacterial resistance to antibiotics.2 Silver nanoparticles (Ag NPs)3 as well as various silver-based compounds and materials containing ionic silver (Ag+)4 or metallic silver (Ag0)5 have recently been synthesized and shown to exhibit antimicrobial activity against several species of bacteria including Escherichia coli.6
E. coli inhabit the intestines of humans and other animals. Although most strains of E. coli are not pathogenic, E. coli O157:H7 produces Shiga-like toxin and causes diarrhea and abdominal cramps,7 and is considered as the most pathogenic strain of E. coli. Outbreaks of E. coli O157:H7 infection have been associated with the consumption of foods of animal origin, such as undercooked beef,8 improperly pasteurized milk,9 or contaminated water.10 It is estimated that these bacteria cause thousands of foodborne illnesses and hundreds of hospitalizations and deaths worldwide each year.11 It is very important to monitor pathogenic E. coli strains and stop their growth by simple and novel means.
Antibacterial activity of silver-containing compounds has been widely used to reduce infections in burn treatment12 and arthroplasty,13 as well as to prevent bacteria colonization on prostheses,14 catheters,15 and human skin.16 Silver ions have also been coated onto stainless-steel materials for use in the food industry17 and water treatment.18 The antimicrobial activity of silver colloids (Ag-c) is influenced by the dimension of the particles, with smaller particles showing greater antimicrobial effect.19
Therefore, in developing the route of synthesis, an emphasis was made to control the size (<10 nm) of Ag NPs. Ag NPs alone in solution easily aggregate, resulting in deterioration of their chemical properties and a loss of their antibacterial properties.20 To solve these problems we synthesized Ag NPs linked with a diamino group (ethylenediamino-propyltrimethoxysilane; EDAPTMS) onto silica NPs (Ag-SiO2) using a modified seed-mediated growth method. Silica particles provide high surface area for surface functionalization, give excellent mechanical strength for supporting Ag NPs, offer thermal stability, and are inexpensive.21 SiO2 has been used previously to grow Ag NPs for improving antibacterial properties of Ag and SiO2 composites. Kim et al20 have grafted Ag NPs directly onto SiO2 NPs with and without using catalyst ammonia solution, and Gao et al21 used quaterized polyethyleneimine-grafted SiO2 NPs.
In our study EDAPTMS was used for surface functionalization of SiO2 NPs to produce a functional composite of silica particles with high nucleophilic properties. Our laboratory is focused on developing low-cost, water-insoluble antibacterial materials for monitoring and improving water quality. Thus, we grafted Ag NPs with EDAPTMS-functionalized SiO2 NPs and studied their antibacterial activity against five different representative bacterial strains as compared to Ag-c NPs22 in aqueous culture media.
Methods
Materials
All the reagents were purchased from Sigma-Aldrich (St. Louis, Missouri), Fisher Scientific (Somerville, New Jersey), and Invitrogen Molecular Probes (Eugene, Oregon), and used without any further purification. Luria Bertani (LB) medium and agar were purchased from EMD Chemicals, Darmstadt, Germany. E. coli strain ATCC 25922 (general strain) and E. coli O157:H7 bovine strains were provided by Dr. Gregory Bohach (University of Idaho, Moscow); Pseudomonas aeruginosa ATCC 27853 and Salmonella enterica ATCC 19585 were obtained from American Type Culture Collection (ATCC; Manassas, Virginia), and Bacillus cereus was obtained from the University of Idaho teaching laboratory for use in this study. Phosphate-buffered saline (PBS) was prepared from a 10× autoclaved stock (10× PBS: 100 mM phosphate buffer, 1.37 M NaCl, 27 mM KCl, pH 7.2. Deionized water (DI; 18.2 MΩ) was collected from Labconco, water Pro PS (Labconco, Kansas City, Missouri).
Instrumentation
A JEOL 1200EX II model (JEOL, Tokyo, Japan) was used for transmission electron microscopy (TEM) images, and a Supera Gemini 35 VP Field Emission-Scanning Electron Microscope (Zeiss, Oberkochen, Germany) coupled with a Thermoelectron (Zeiss) model was used for field emission scanning electron microscopy (FE-SEM) imaging. The functional group of the nanoparticles was characterized using an ultraviolet-visible (UV-vis) spectrophotometer model Pharma-Spec UV-1700 (Shimadzu, Tokyo, Japan). Bacterial growth was monitored using a Beckman Coulter LD 400 luminometer (Beckman Coulter, Fullerton, California) along with AD LD analysis software version 1.6 (VWR International, West Chester, Pennsylvania). Bactericidal effects were characterized using a Leitz LaborLux S microscope (VWR International, West Chester, Pennsylvania) with a Leica EC3 objective head and camera (Leica Microsystems Inc., Bannockburn, Illinois) and a Leo fluorescent Lamp (VWR International, West Chester, Pennsylvania). All glassware was cleaned with aqua-regia and rinsed with ethanol and DI water several times before use.
Synthesis of Ag-SiO2 and Ag-c NPs
The Ag-SiO2 NPs were synthesized from their monomer precursor, tetraethoxysilane, using a slightly modified seed-mediated growth method.23 The silica NPs were prepared by mixing 3.0 mL of 28–30% (vol/vol) ammonium hydroxide solution, 0.5 mL DI water, and 50.0 mL of ethanol. The silica precursor (1.5 mL of tetraethoxysilane) was added to the above solution dropwise (50 μL/min) over 30 minutes and stirred at 1200 rpm overnight at room temperature (RT, 20–22°C). As the silica NPs formed, the solution became opaque. Then SiO2 NPs were separated using centrifugation at a speed of 4000 rcf and washed with ethanol three times. This was followed by suspending the NPs in 50 mL of ethanol and treating with 5% EDAPTMS for surface functionalization.24 The reaction mixture was stirred at RT for 4 hours and refluxed for 1 hour at 80°C; then 0.5 mL of 1% (wt/vol) sodium citrate (Na3C6H5O7) solution and 500 μL of 0.05 M AgNO3 were added dropwise. The reaction mixture was stirred for 1 hour, and the color changed from opaque to light brown. The NPs were separated from solution by centrifuging at 4000 rcf for 30 minutes and washed three times with 50% (vol/vol) ethanol/water. The NPs were then dried overnight in vacuum desiccators and cured at 110°C for 12 hours in a conventional oven. NPs were cooled to RT and stored at 4°C.
The Ag-c NPs were prepared in our laboratory as reported by Reddy et al.25 Briefly, 5 mL of a 5 mM AgNO3 solution were added to 20 mL of water giving 25 mL of a 1 mM AgNO3 solution. The solution was placed in a 50-mL conical flask and stirred at 1200 rpm and heated until it began to boil. Then, 1 mL of 1% (wt/vol) sodium citrate solution was added at once. Vigorous stirring and heating continued until a color change to pale yellow was visible (at 3.5 minutes). The solution was then removed from the heating plate but stirring continued while cooling to RT. The solution was topped with water up to 25 mL to account for boiling losses. The pale yellow solution was stored at 4°C.
The presence of Ag NPs on Ag-SiO2 NPs was confirmed using UV-vis absorbance spectrometry. Size distribution and morphology of Ag-SiO2 and Ag-c NPs were characterized using TEM imaging.
Bacterial growth-inhibiting effect in LB broth using turbidity
E. coli strains were cultured on LB agar plates for 18 hours at 37°C before use. Differing concentrations of Ag-c and Ag-SiO2 NPs were prepared in sterilized LB medium in a final volume of 10 mL. The growth of bacteria was monitored with and without Ag-c at concentrations of 0.085, 0.85, 8.5, 17, 34, 42.5 μg/mL, and Ag-SiO2 NPs at 0.1, 1, 10, and 100, μg/mL. A single colony of E. coli was used for inoculating the LB medium containing the NPs as well as a positive control containing only bacteria without Ag-c NPs. Aliquots were taken every hour up to 9 hours and then after 24 hours for measurement of the optical density (OD) at 620 nm.
Bacterial growth-inhibiting effect based on LB agar plating
The growth-inhibiting effects of Ag-c and Ag-SiO2 NPs were further confirmed by plating on LB agar plates after 24 hours of growth at 37°C in LB broth. Serial dilutions of these mixtures were made in PBS up to 10−9 concentration and then plated on LB agar plates. Samples containing only bacteria were plated as positive controls. The LB broth containing E. coli and NPs was diluted based on the growth that was observed through OD readings. The plates were incubated at 37°C for 24 hours followed by counting the number of colonies on the plate and calculating the increase in colony-forming units (cfu) per milliliter according to the following formula26: [(Viable count at 0 hours − Viable count at 24 hours)/Viable count at 0 hours] × 100%.
Bactericidal effect using live/dead cell staining
Experiments were carried out in the presence and absence of silver NPs to determine the cell viability of E. coli and E. coli O157:H7 bacteria by using the BacLight27 bacterial viability kit (Invitrogen, Carlsbad, California). E. coli–inoculated LB broth with and without Ag-SiO2 or Ag-c NPs was incubated at 37°C for 24 hours. The samples were centrifuged at 3500 rcf for 10 minutes so as to pellet the cells and then rinsed three times with 1 mL sterile 0.85% NaCl solution. After the final rinse the cells were resuspended in 1 mL 0.85% NaCl solution, and 1 μL of each of reagents A and B from the kit were added. The suspension was incubated at RT for 15 minutes followed by filtration onto a 25-mm black polycarbonate filter, so as to concentrate the cells. The filter was then placed onto glass microscope slides with coverslips and visualized under a fluorescent microscope using red and green filters for analysis. The dead cells were counted using a red filter, and the live cells were counted using the green filter.
Antibacterial test
The antimicrobial activity of Ag-SiO2 and Ag-c NPs was tested using the standard microdilution method,28 which determines the minimum inhibitory concentration (MIC) leading to the inhibition of bacterial growth. Five different bacteria (E. coli ATCC 25922, E. coli O157:H7, Pseudomonas aeruginosa ATCC 27853, Salmonella enterica ATCC 19585, and Bacillus cereus were tested at a concentration of ~107, 107, 108, 105, and 106 cfu/mL, respectively. Bacteria were grown on LB agar broth with five different concentrations of Ag-SiO2 (10, 50, 75, 100, 125 μg/mL) and Ag-c (8.5, 17.0, 34, 42.5, 85 μg/mL). The samples were incubated for 24 hours at 35°C, and then 100-μL samples were placed on nutrient agar plates. The MIC values were read after 24 hours of incubation at 35°C.
Results
Characterization of the synthesized Ag-SiO2 and Ag-c nanoparticles
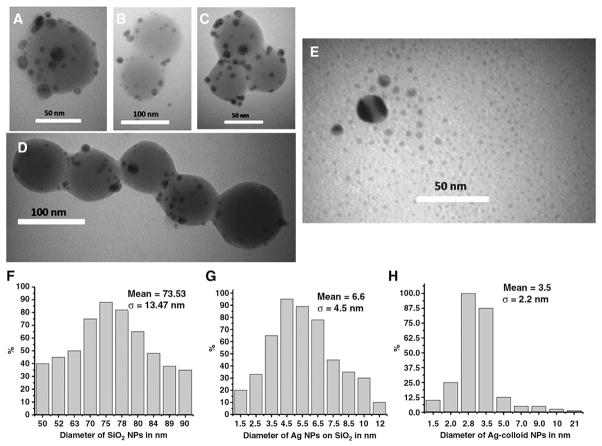
The Ag-SiO2 NPs used in this study were imaged by TEM (Figure 1, A–D). The shape of these silica NPs was nearly spherical, and their average diameter (Figure 1, F) was ~73.53 ± 13.47 nm. The silver particles linked to the surface of SiO2 NPs (Figure 1, A–D) are also nearly spherical in shape with a mean size of 6.6 ± 4.5 nm (Figure 1, G). The TEM image of Ag-c NPs (Figure 1, E) showed an average size of about 3.5 ± 2.2 nm (Figure 1, H). Analysis of the TEM images of both particles reveals that the average sizes of Ag NPs, both free and on the surface of SiO2, are in the nanometer range.
Figure 1.
TEM images (A–D) of Ag-SiO2 NPs and (E) Ag colloids NPs. (F–H) Size distribution graphs of (F) SiO2 NPs (mean size = 73.53 nm), (G) Ag NPs linked with SiO2 (mean size = 6.6 nm), and (H) Ag-c NPs (mean size = 3.5 nm).
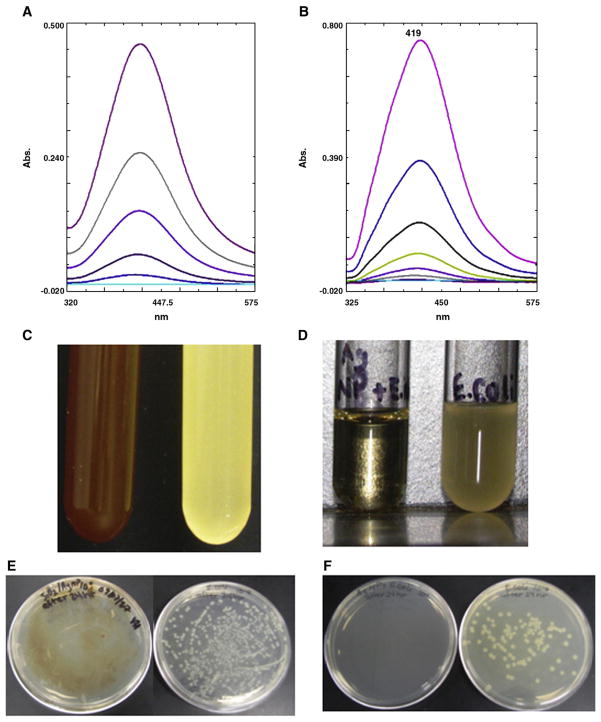
The prepared aqueous solution of Ag-SiO2 and Ag-c NPs shows an absorption peak at 390–420 nm in the UV-vis spectrum (Figure 2, A and B, respectively). This is a typical absorption band of crystalline spherical Ag NPs29 and is due to their surface plasmon.30 The stability of a concentrated solution was checked by observing the absorption spectrum after serial dilutions ranging from five to seven times. The absorption spectrum of the diluted NPs is almost identical to the spectral features of a concentrated solution of Ag-SiO2 NPs. This confirms that the Ag-SiO2 NPs are not further dimerized or agglomerated.31 However, in the case of Ag-c NPs solutions the absorption spectrum at higher concentrations is different from solutions of lower concentration, which indicates that there are some particles that are dimerized or agglomerated.
Figure 2.
UV-visible spectra of (A) aqueous suspension of Ag-SiO2 NPs at 1000, 100, 10, 1, and 0.1 μg/mL (top to bottom) and (B) aqueous solution of Ag-c NPs at 85, 42.5, 8.5, 0.85, 8.5 × 10−2, and 8.5 × 10−3 μg/mL (top to bottom). (C, D) Antibacterial effect in culture tube after 24 hours’ incubation of (C) Ag-SiO2 NPs (lefthand tube contains 100 μg/mL Ag-SiO2 NPs and E. coli, and righthand tube contains only E. coli control); (D) Ag-c NPs (lefthand tube contains 42.5 μg/mL Ag-c NPs with E. coli, and righthand tube contains only E. coli control). (E, F) Growth test of E. coli on agar plates. (E) Lefthand plate contains Ag-SiO2 NPs + E. coli, and righthand plate contains only E. coli control, which contains ~1.78 × 106 cfu of E. coli. (F) Lefthand plate contains Ag-c NPs + E. coli, and righthand plate contains only E. coli control, which contains ~1.28 × 106 cfu of E. coli.
Antibacterial activity of Ag-SiO2 and Ag NPs against E. coli
The different concentrations of Ag-SiO2 and Ag-c NPs were mixed with E. coli in culture tubes and incubated 24 hours at 37°C. Bacterial growth was totally suppressed in the presence of both kinds of Ag NPs (Figure 2, C and D). This was confirmed by plating a diluted aliquot from each tube onto a nutrient agar plate and observing after incubation for 24 hours at 37°C. Bacterial growth in the presence of Ag-SiO2 NPs (Figure 2, E) and controls and in the presence of Ag-c NPs shows the same results (Figure 3, F).
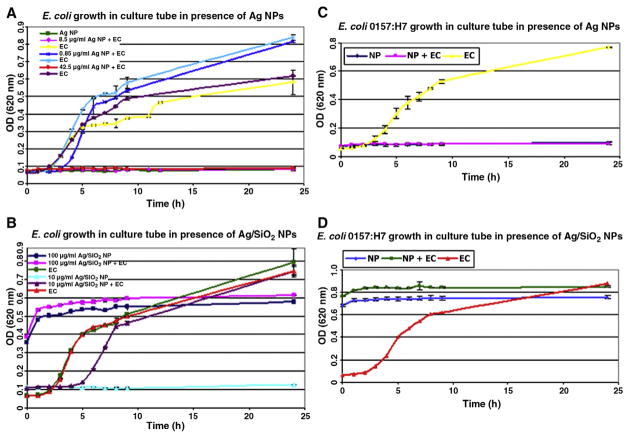
Figure 3.
Growth curve of E. coli (EC) ATCC 25922 (A) in presence of Ag-c NPs at a concentration of 0.85 (dark blue), 8.5 (pink), and 42.5 μg/mL (red) with their positive controls (bacteria only) light blue, yellow, and violet, respectively, and negative control Ag-c NPs only (green line); (B) in presence of Ag-SiO2 NPs at a concentration of 100 (pink) and 10 (violet) μg/mL, with bacteria only (green and red) and Ag-SiO2 NPs only (dark blue and light blue), respectively. (C, D) Growth curve for E. coli O157:H7 (C) in presence of Ag-c NPs at concentration of 42.5 μg/mL (pink), bacteria only (yellow), and NPs only (blue); (D) in presence of Ag-SiO2 NPs at concentration of 100 μg/mL (green), bacteria only (red), and NPs only (light blue).
To test the antibacterial effectiveness of these NPs against general E. coli and E. coli O157:H7, the OD was measured in LB broth in the presence and absence of NPs. A level of 8.5 or 42.5 μg/mL of Ag-c NPs totally inhibited the growth of the general strain of E. coli throughout the 24-hour period of incubation (Figure 3, A). Limited inhibition was observed at 8.5 μg/mL. The Ag-SiO2 NPs at 10 μg/mL inhibited the growth of the general E. coli strain up to 6 hours, whereas 100 μg/mL was effective throughout the 24-hour incubation and totally prevented growth (Figure 3, B). As is noted, 100 μg/mL Ag-SiO2 NPs control did result in a high initial turbidity, but there was no apparent change over time, indicating inhibition of bacterial growth. In tests against E. coli O157:H7, the higher concentrations of Ag-c (42.5 μg/mL) and Ag-SiO2 (100 μg/mL) NPs also inhibited the growth up to 24 hours (Figure 3, C and D, respectively). The complex of E. coli and Ag-SiO2 NPs was analyzed using FE-SEM imaging (Figure 4, C and D).
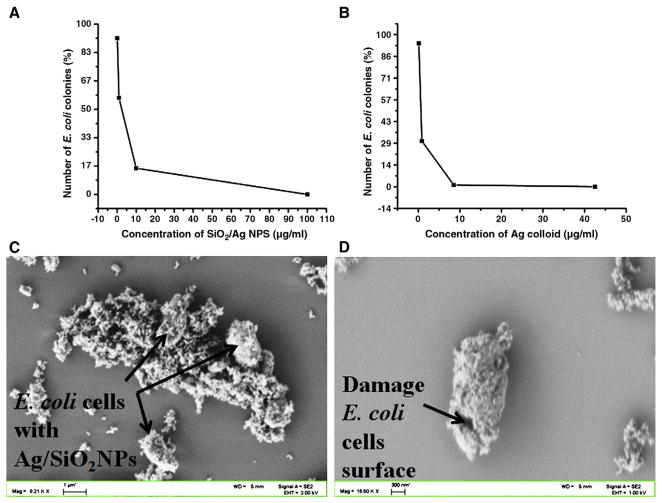
Figure 4.
Colonies count of E. coli ATCC 25922 expressed as a percentage of the number of colonies grown on silver-free LB agar plates. (A) Ag-SiO2 NPs at 0.1, 1, 10, and 100 μg/mL; (B) colloidal Ag NPs at 0.085, 0.85, 8.5, and 42.5 μg/mL. (C, D) FE-SEM images of (C) damaged E. coli ATCC 25922 and Ag-SiO2 NPs complex; (D) damaged cell surface of single E. coli bacterium in presence of Ag-SiO2 NPs.
Plate counts were also used to monitor growth of the general strain of E. coli at the end of the 24-hour incubation. The inhibitory effects observed using turbidity were confirmed using plate counts. It should be pointed out that the samples with NPs were diluted before plating; however, the NPs were still present in the plates and thus probably influenced growth on the plates. Concentrations of 5 and 10 μg/mL of Ag-SiO2 and 8.5 μg/mL Ag-c NPs reduced growth of E. coli on the plates, but no growth was observed at 100 μg/mL of Ag-SiO2 and at 42.5 μg/mL of Ag-c NPs (Figure 4, A and B, respectively). These results confirm that the synthesized Ag-SiO2 NPs have antibacterial effectiveness similar to Ag-c NPs.
Live-dead monitoring of bacterial cells
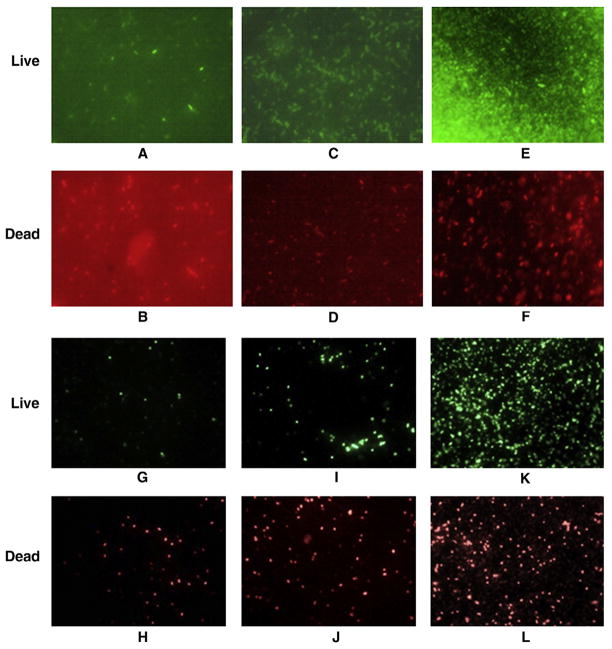
The fluorescence images of the stained bacterial cells were taken using a microscope with a green filter (535 nm) used for visualizing live cells and a red filter (642 nm) used for dead cells (Figure 5, A–L. All the fluorescent images were captured from a single spot of each sample by flipping the red and green filter set, which gives reliable digital counting of cells.
Figure 5.
Fluorescent microscopy images of live and dead bacterial cells in presence of silver NPs, using green and red UV-visible filters, respectively. (A, B) 100 μg/mL Ag-SiO2 NPs + E. coli ATCC 25922; (C, D) 8.5 μg/mL Ag colloid NPs + E. coli ATCC 25922; (E, F) control having only E. coli ATCC 25922; (G, H) 100 μg/mL Ag-SiO2 NPs + E. coli O157:H7; (I, J) 8.5 μg/mL Ag colloid NPs + E. coli O157:H7; (K, L) control containing only E. coli O157:H7.
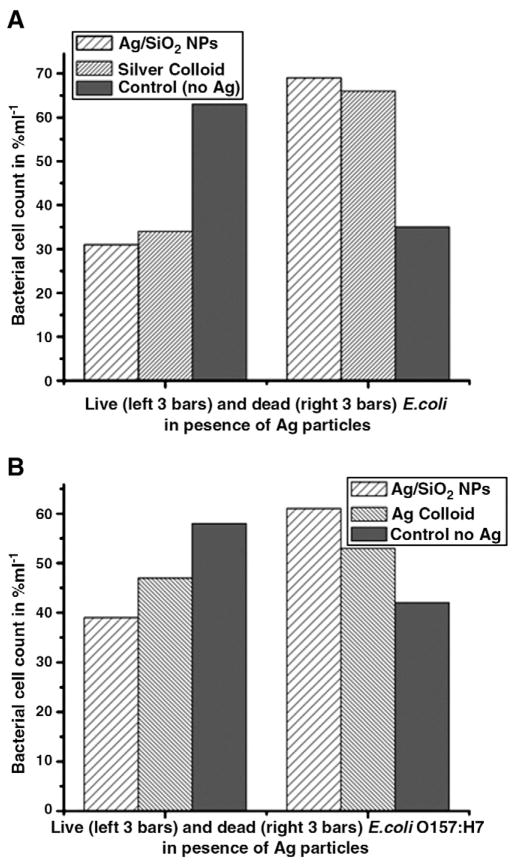
The digital counting of live vs. dead bacterial cells was expressed as the function of percentage, and results are the mean of three images. In the presence of 100 μg/mL of Ag-SiO2 NPs, 74% of the general strain were killed, whereas 64% of E. coli O157:H7 were killed (Figure 6, A and B). Ag-c NPs at 8.5 μg/mL showed similar results, with a 70% death of the general strain and 60% death of E. coli O157:H7.
Figure 6.
Bar graph of live and dead (A) E. coli ATCC 25922, (B) E. coli O157:H7 in the presence and absence of Ag-c NPs, where bacterial cells counts are expressed as a function of percentage per milliliter.
The MIC values of synthesized Ag-SiO2 and Ag-c NPs are shown in Table 1 against five different bacteria.
Table 1.
Minimum inhibitory concentrations for Ag-SiO2 and Ag-c NPs against five different bacterial strains
| Bacteria | Ag-SiO2 NPs (μg/mL) | Ag-c NPs (μg/mL) |
|---|---|---|
| E. coli ATCC 25922 | 125 | 34 |
| E. coli O157:H7 | 125 | 34 |
| Pseudomonas aeruginosa ATCC 27853 | 125 | 34 |
| Salmonella enterica ATCC 19585 | 125 | 42.5 |
| Bacillus cereus | 150 | 42.5 |
Discussion
We prepared silica NPs using a variation of the method developed by Stöber et al32 with the surface functionalized with EDAPTMS. The TEM analysis (Figure 1, A–D) clearly shows attachment of Ag NPs on SiO2, and the Ag-SiO2 composites were observed individually as well as in small aggregates. In our previous study we reported that the surface functionalization of silica NPs with amino-propyltrimethoxysilane (APTMS) was useful for linking with fluorescein dye.33 The premodification of silica with EDAPTMS provides two nucleophilic (amino) groups that immobilize the metallic silver particles on the surface of silica particles. Thus, silver NPs of small size were tethered to the EDAPTMS-functionalized silica surface by virtue of the aminophilic nature of silver particles. These small colloidal particles coordinate to lone pairs of the primary and secondary amino groups of EDAPTMS, which stabilizes the Ag NPs on the surface of silica. As is well known, the alkyl-amines exist predominantly as positive-charged R-NH3+ groups when the value of pH is below 10 (Ref. [34]), and the reduction of Ag+ with excess sodium citrate afforded small silver particles with a net negative interfacial charge.35 Due to electrostatic repulsive force, the silver particles immobilized on the surface are separated, as is shown in the TEM image of Ag-SiO2 particles.
The OD graph and agar plate readings of well-dispersed Ag-c NPs at 42.5 μg/mL and Ag-SiO2 at 100 μg/mL shows similar antibacterial effectiveness in LB broth against E. coli ATCC 25922 and E. coli O157:H7. The mechanism of antibacterial property of Ag NPs has been well established by free-radical generation from Ag NPs using electron spin resonance spectroscopy,31 and O-Ag vibration at 206–232 cm−1 and ionic species adsorbed onto Ag NPs were observed using surface-enhanced Raman spectroscopy (SERS).36 A similar SERS peak at 232 cm−1 has been observed after treatment of E. coli with synthesized Ag NPs in our laboratory (data not shown), but the FE-SEM images (Figure 4, C and D) clearly show that the toxic effect of Ag-SiO2 NPs results from, damage to the cell surface of E. coli.
In the presence of 10 μg/mL of SiO2-Ag NPs, growth of both E. coli ATCC 25922 and E. coli O157:H7 was inhibited, and at 100 μg/mL, the bacteria did not grow even after 24 hours. At an Ag-SiO2 NPs concentration of 100 μg/mL, 74% of the general E. coli and 64% of E. coli O157H7 were killed. However, at an Ag-c NP concentration of 8.5 μg/mL, 71% of the general E. coli ATCC 25922 were killed and 60% of E. coli O157:H7.
Furthermore, the broad spectrum of antibacterial properties of Ag-SiO2 and Ag-c NPs was shown with both gram-negative and gram-positive bacteria. MIC values show that 125 μg/mL Ag-SiO2 and 34 μg/ml Ag-c were sufficient to inhibit gram-negative bacteria [E. coli ATCC 25922, E. coli O157:H7, Pseudomonas aeruginosa ATCC 27853, Salmonella enterica ATCC 19585 (42.5 μg/mL; Ag-c NPs)], whereas 150 μg/mL Ag-SiO2 and 42.5 μg/mL Ag-c were required to inhibit the gram-positive bacterium (Bacillus cereus). This difference could be due to the different bacterial concentrations as well as type of strain used.
In conclusion, Ag-SiO2 and Ag-c NPs prepared by the cost-effective reduction method described here show great promise as antimicrobial agents against both gram-negative and gram-positive bacteria. Applications of these NPs based on our findings may lead to valuable discoveries in various fields such as improving water quality, medical devices, and antimicrobial systems.
Acknowledgments
The authors would like to thank the Electron Microscopy Center Facility at the University of Idaho (UI), Moscow, for providing TEM and FE-SEM imaging of nanoparticles, and also the Center for Biological Applications of Nanotechnology (BANTech) group of UI.
This study was supported by IDeA Networks of Biomedical Research Excellence/National Institutes of Health (P20RR16454) and U.S. Department of Agriculture (2006-34479-17058).
References
- 1.Khaydarov RR, Khaydarov RA, Estrin Y, Evgrafova SE, Scheper T, Endres C, et al. Nanoparticles risk and benefits. Dordrecht, The Netherlands: Springer; 2008. [Google Scholar]
- 2.Vlieghe E, Phoba MF, Muyembe Tamfun JJ, Jacob J. Antibiotic resistance among bacterial pathogens in central Africa: a review of the published literature between 1955 and 2008. Int J Antimicrob Agents. 2009;34:295–303. doi: 10.1016/j.ijantimicag.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Jo SC, Rim AR, Park HJ, Yuk HG, Lee SC. Combined treatment silver ions and organic acid enhances growth-inhibition of Escherichia coli O157:H7. Food Control. 2009;46:296–9. [Google Scholar]
- 5.Lee D, Cohen RE, Rubner MF. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir. 2005;21:9651–9. doi: 10.1021/la0513306. [DOI] [PubMed] [Google Scholar]
- 6.Panáček A, Kvitek L, Prucek R, Kolář M, Večeřová R, Pizǔrová N, et al. Silver colloid nanoparticles: synthesis, characterization and their antibacterial activity. J Phys Chem B. 2006;110:16248–53. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Q, Li L, Guo Z, Yang R. Identification of Shiga-like Escherichia coli isolated from children with diarrhea by polymerase chain reaction. Chin Med J. 2002;115:815–8. [PubMed] [Google Scholar]
- 8.Ochoa ML, Harrington PB. Immunomagnetic isolation of enterohemor-rhagic Escherichia coli O157:H7 from ground beef and identification by matrix assisted laser desorption/ionization time-of-flight mass spectrometry and databases searches. Anal Chem. 2005;77:5258–68. doi: 10.1021/ac0502596. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Ye M, Chao Q, Jia N, Ge Y, Shen H. Simultaneous detection of multifood-food borne pathogenic bacteria based on functionalized quantum dots coupled with immunomagnetic separation in food samples. J Agric Food Chem. 2009;57:517–24. doi: 10.1021/jf802817y. [DOI] [PubMed] [Google Scholar]
- 10.Miles SL, Gerba CP, Pepper IL, Reynolds KA. Point-of-use drinking water devices for assessing microbial contamination in finished water and distribution system. Environ Sci Technol. 2009;43:1425–9. doi: 10.1021/es801482p. [DOI] [PubMed] [Google Scholar]
- 11.Muniesa M, Jofre J, García-Aljaro C, Blanch AR. Occurrence of Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli in the environment. Environ Sci Technol. 2006;40:7141–9. doi: 10.1021/es060927k. [DOI] [PubMed] [Google Scholar]
- 12.Ülkür E, Oncul O, Karagoz H, Yeniz E, Celiöz B. Comparison of silver-coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigrass), and fusidic acid 2% (Fucidin) for optical antibacterial effect in methicillin-resistant Staphylococci-contaminated, full skin thickness rat burn wounds. Burns. 2005;31:874–7. doi: 10.1016/j.burns.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Alt V, Bechert T, SteinrÜcke P, Wagener M, Seidel P, Dingeldein E, et al. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–91. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 14.Gosheger G, Hardes J, Ahrens H, Streiburger A, Buerger H, Erren M, et al. Silver-coated megaendoprostheses in a rabbit model—an analysis of the infection rate and toxicological side effects. Biomaterials. 2004;25:5547–56. doi: 10.1016/j.biomaterials.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Rupp ME, Fitzgerald T, Marion N, Helget V, Puumala S, Anderson JR, et al. Effect of silver-coated urinary catheters: efficacy, cost-effectiveness, and antimicrobial resistance. Am J Infect Control. 2004;32:445–50. doi: 10.1016/j.ajic.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Lee HJ, Jeong SH. Bacteriosis and skin innoxiousness of nanosize silver colloids on textile. Text Res J. 2005;75:551–6. [Google Scholar]
- 17.Yang W, Shen C, Ji Q, An H, Wang J, Liu Q, et al. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology. 2009:20, 085107, 7. doi: 10.1088/0957-4484/20/8/085102. [DOI] [PubMed] [Google Scholar]
- 18.Pradeep T, Jain P. Potential of silver nanoparticles-coated polyure-thane foam as an antibacterial water filter. Biotechnol Bioeng. 2005;90:59–63. doi: 10.1002/bit.20368. [DOI] [PubMed] [Google Scholar]
- 19.Fernando RH. Nanocomposites and nanostructured coatings: recent advancements. Nanotechnol Appl Coat ACS Symp Ser. 2009;1008:2–21. [Google Scholar]
- 20.Kim YH, Lee DK, Cha HG, Kim CW, Kang YS. Synthesis and characterization of antibacterial Ag-SiO2 nanocomposite. J Phys Chem C. 2007;111:3629–35. [Google Scholar]
- 21.Gao B, Zhang X, Wang J. Preparation and antibacterial characteristic of water-insoluble antibacterial material QPEI/SiO2. J Mater Sci Mater Med. 2008;19:3021–8. doi: 10.1007/s10856-008-3428-z. [DOI] [PubMed] [Google Scholar]
- 22.Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed Nanotechnol Biol Med. 2007;3:168–71. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Jiang ZJ, Liu CY. Seed-mediated growth technique for the preparation of silver nanoshell on a silica sphere. J Phys Chem B. 2003;107:12411–5. [Google Scholar]
- 24.Jiang ZJ, Liu CY, Sun LW. Catalytic properties of silver nanoparticles supported on a silica sphere. J Phys Chem B. 2005;109:1730–5. doi: 10.1021/jp046032g. [DOI] [PubMed] [Google Scholar]
- 25.Reddy VR, Currao A, Calzaferri G. Gold and silver metal nanoparticle-modified AgCl photocatalyst for water oxidation to O2. J Phys Conf Ser. 2007;61:960–5. doi: 10.1002/cphc.200301211. [DOI] [PubMed] [Google Scholar]
- 26.Maneerung T, Tokura S, Rujirvanit R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym. 2008;72:43–51. [Google Scholar]
- 27.Choi O, Deng KK, Kim NJ, Ross JL, Surampalli RY. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008;42:3066–74. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Castañón GA, Niño-Martínez N, Martínez-Gutierrez F, Martínez-Mendoza JR, Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res. 2008;10:1343–8. [Google Scholar]
- 29.Shirtcliffe N, Nicle U, Schneider S. Reproducible preparation of silver sols with small particle size using borohydride reduction: for use as nuclei for preparation of larger particles. J Colloid Interface Sci. 1999;211:122–9. doi: 10.1006/jcis.1998.5980. [DOI] [PubMed] [Google Scholar]
- 30.Creighton JA, Blatchfor CG, Albrecht MG. Plasmaresonance enhancement of Raman scattering by pyridine adsorbed on silver or gold particles size comparable to the excitation wavelength. J Chem Soc Faraday Trans II. 1979;75:790–8. [Google Scholar]
- 31.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol Biol Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Stöber W, Fink A, Bohn E. Controlled growth of monodispersed silica spheres in micron size range. J Colloid Interface Sci. 1968;26:62–9. [Google Scholar]
- 33.Rastogi SK, Hendricks VJ, Branen JR, Branen AL. Magnetic bead and fluorescent silica nanoparticles based optical immunodetection of staphylococcal enterotoxin B in bottled water. Sensors & Transducers J1726-5479. 2009;7:191–202. [Google Scholar]
- 34.Westcott SL, Oldenburg SJ, Lee RL, Halas NJ. Formation and adsorption of clusters of gold nanoparticles onto functionalized silica nanoparticles surfaces. Langmuir. 1998;14:5396–401. [Google Scholar]
- 35.Ung T, Liz-Marzán LM, Mulvaney P. Redox catalysis using Ag@SiO2 colloid. J Phys Chem B. 1999;103:6770–3. [Google Scholar]
- 36.Naja G, Bouvrette P, Champagne J, Brousseau R, Luong JHT. Activation of nanoparticles by biosorption for E. coli detection in milk and apple juice. Appl Biochem Biotechnol. 2010;162:460–75. doi: 10.1007/s12010-009-8709-6. [DOI] [PubMed] [Google Scholar]