Fig. 5. The PTD-p65 (38–47) peptide blocks the recruitment of RelA/p65 and DNMTs to the BRMS1 promoter.
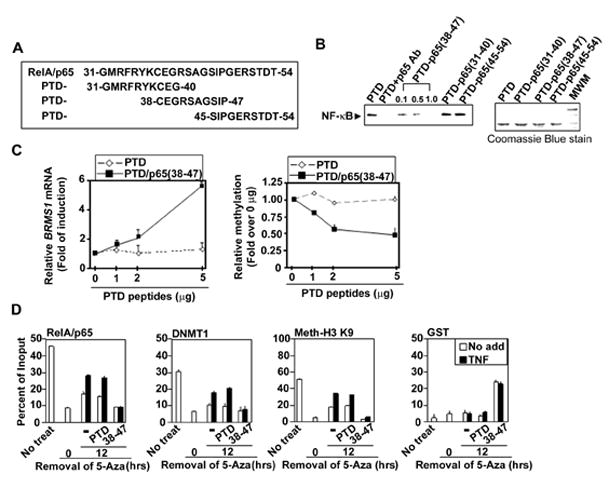
(A) Diagram of protein transduction domain (PTD) peptides conjugated with the putative RelA/p65 DNA binding regions. (B) The PTD-p65 (38–47) peptide inhibits NF-κB binding to the BRMS1 promoter. EMSAs were performed using 293T cell nuclear extracts incubated with indicated peptides (1μg) and 33P-labeled -κB binding site II of BRMS1 promoter as the probe. (C) PTD-p65 (38–47) peptide increases BRMS1 transcripts in NSCLC cells. (Left) BRMS1 mRNA was analyzed by quantitative RT-PCR and (Right) the relative methylation of BRMS1 promoter was evaluated by quantitative MSP in H157 cells treated with PTD peptides at indicated doses for 24 hrs. (D) Inhibition of RelA/p65 DNA binding reduces chromatin-associated DNMT-1 on the BRMS1 promoter. H157 cells were treated with 5-Aza as described previously. After removal of 5-Aza, cells were treated with indicated peptides (5μg/ml) and TNF (20ng/ml) for additional 24 hrs. ChIP analysis was performed.
