Abstract
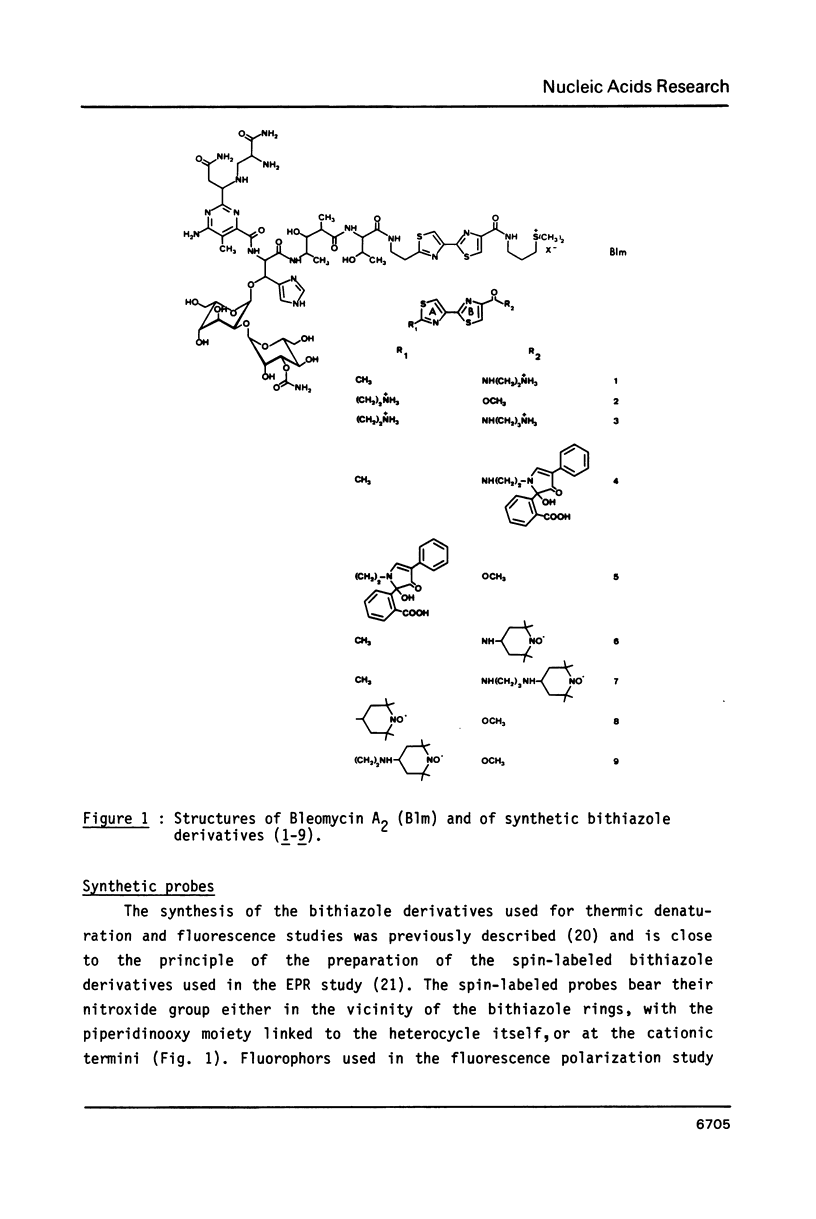
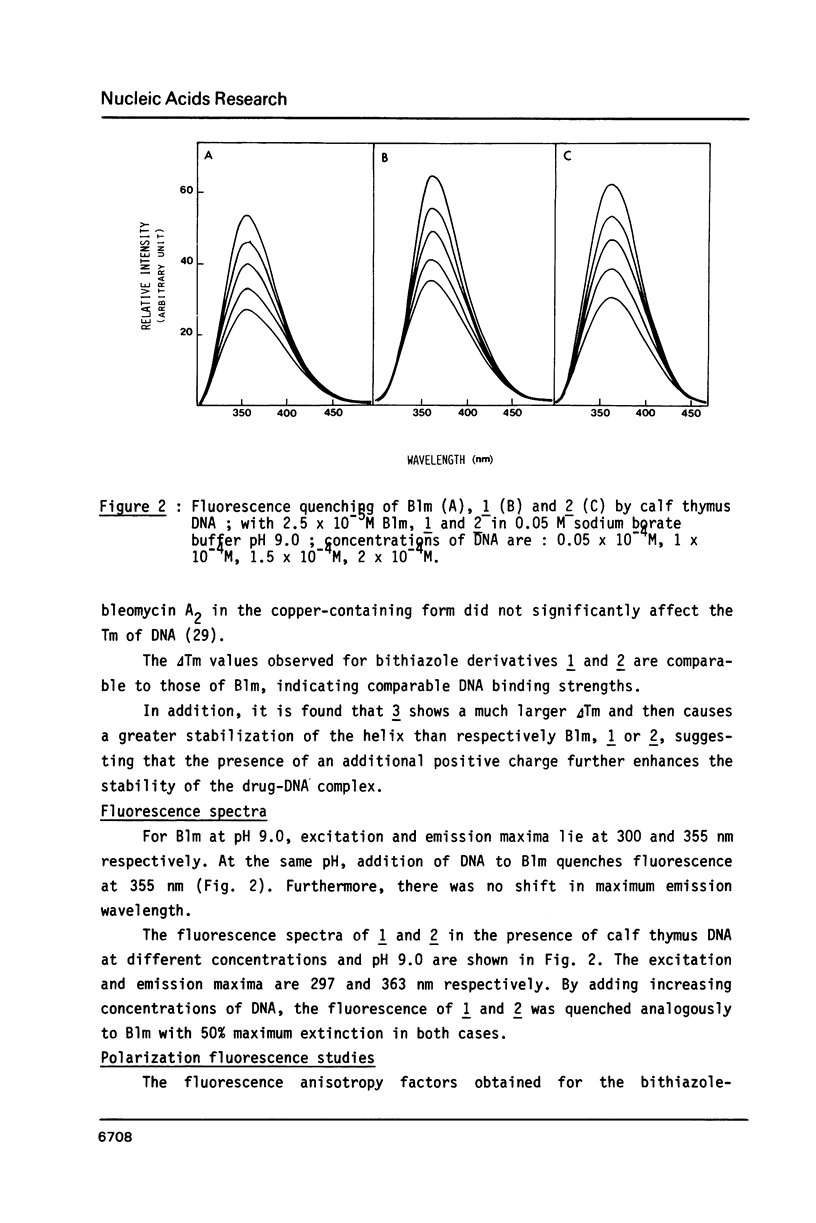
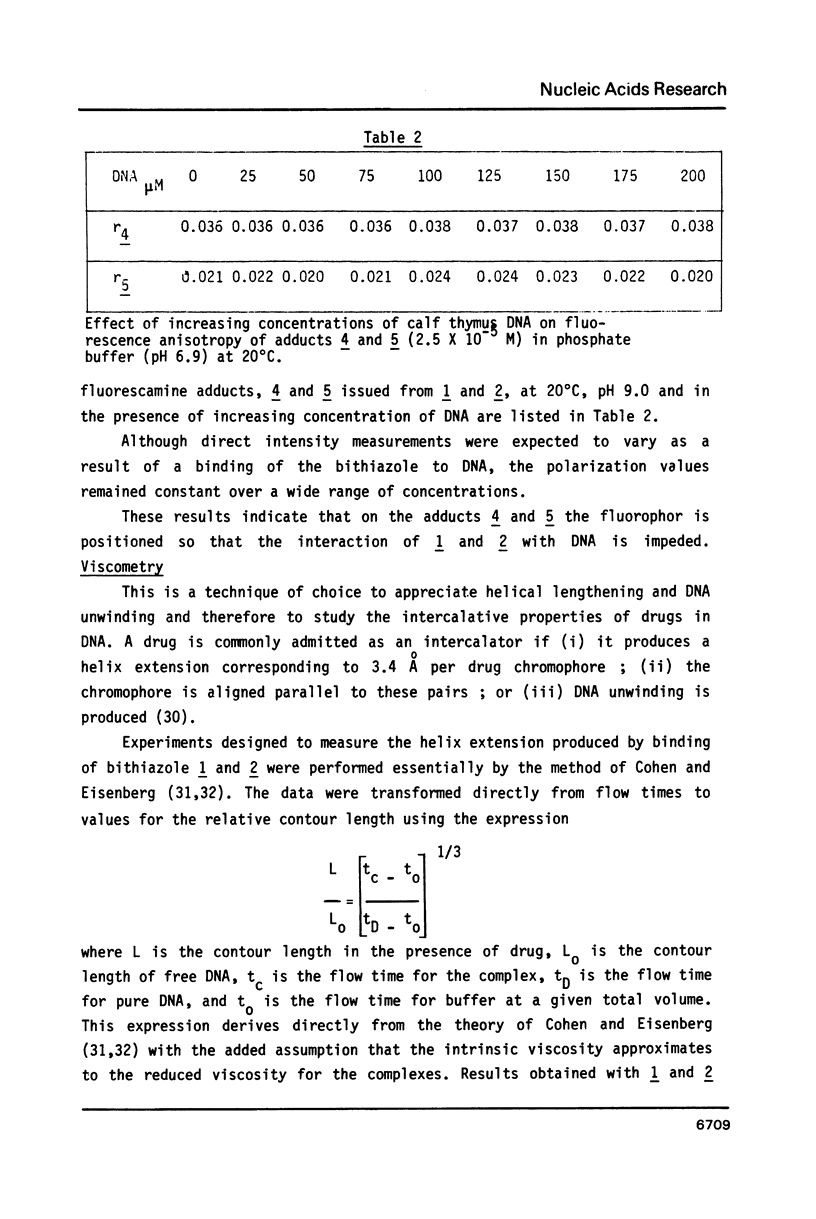
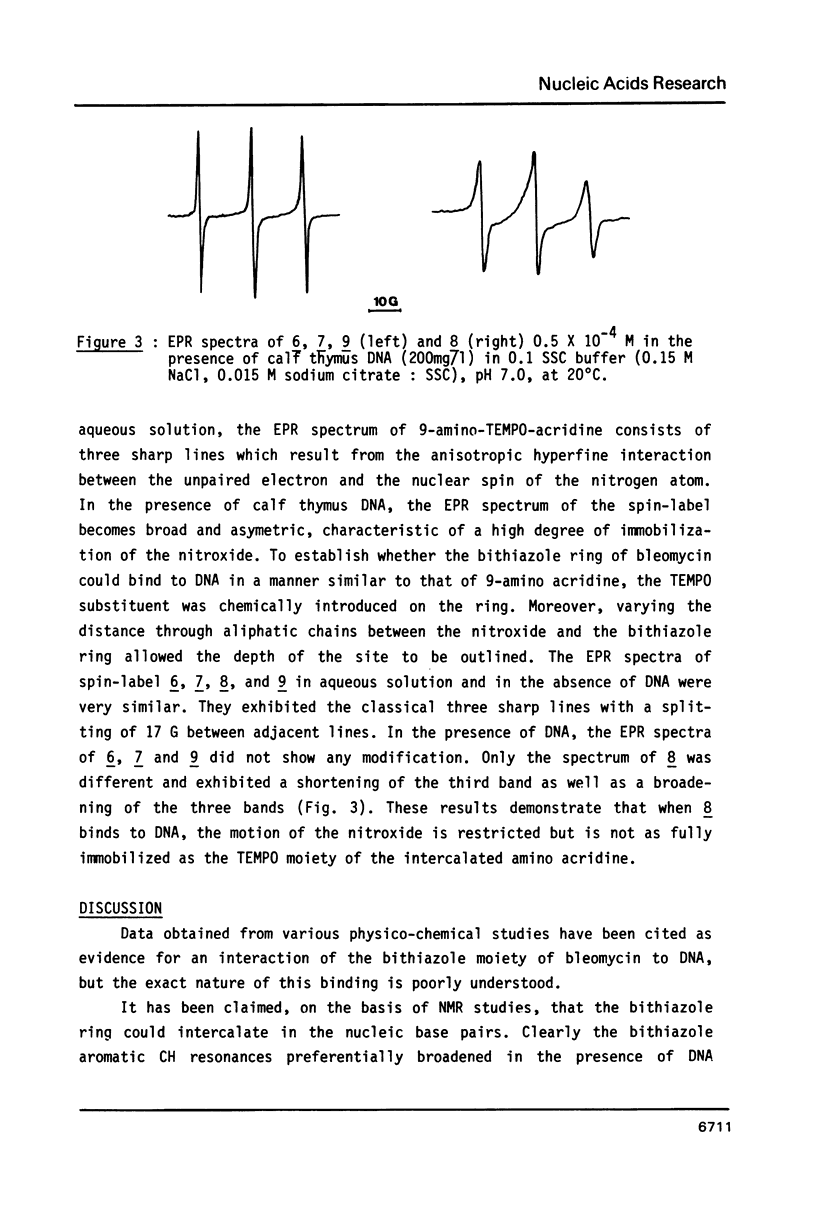
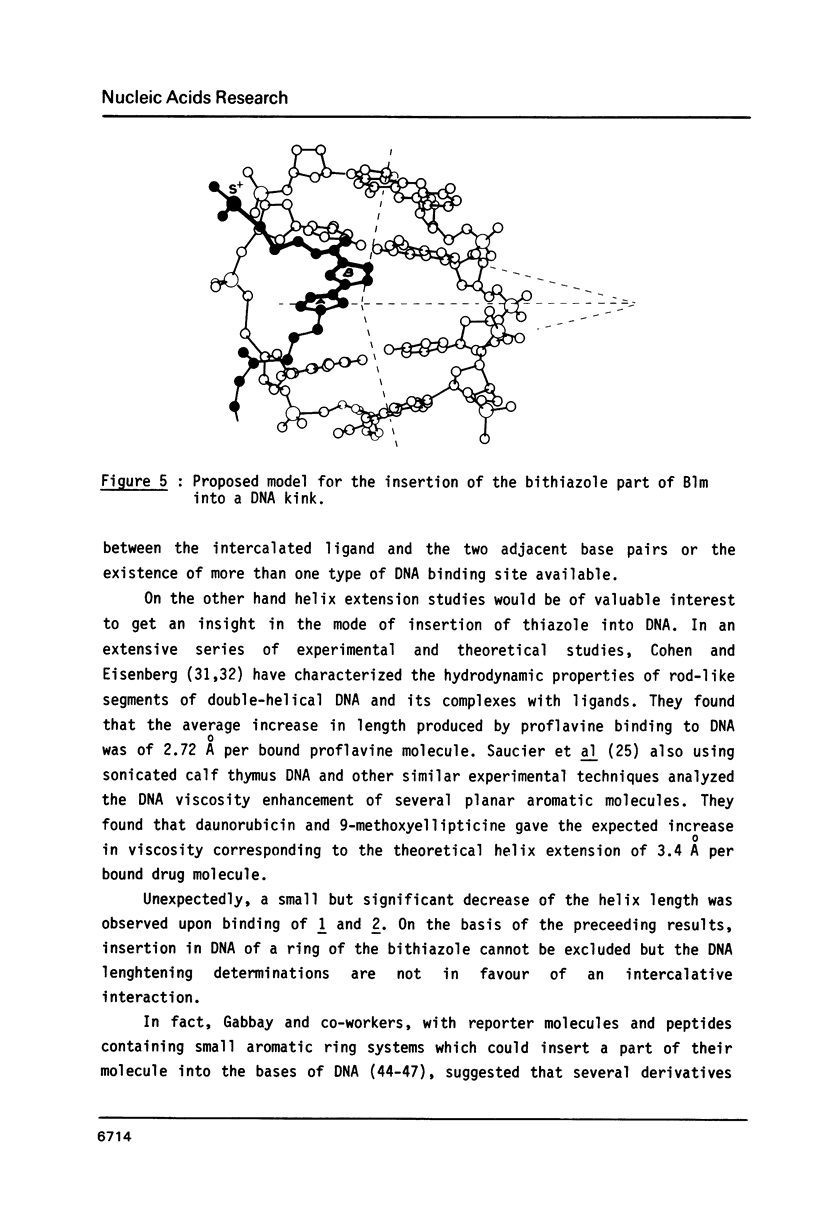
Bleomycin is a widespread anticancerous drug, the biological activity of which having been extensively studied. Its metal ion-chelating portion has been shown to cleave DNA whereas the role of the bithiazole moiety is still questionable. In order to elucidate this problem some 2', 4-disubstituted bithiazoles structurally related to the "tripeptide S" moiety of bleomycin were synthesized and their interaction with DNA was studied using delta Tm, fluorescence, EPR and viscometry techniques. The results of delta Tm and fluorescence quenching determinations were in favour of a binding of the bithiazole part by an intercalation process. Nevertheless, the use of the spin-label probes indicated only a partial intercalation of the ring between the base-pairs. Moreover, viscometry data which clearly exhibited a slight decrease of DNA length in the presence of bithiazole derivative led to the proposal of a binding model involving a partial insertion of a thiazole ring which wedges in between the bases at a bending point of DNA.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adawadkar P., Wilson W. D., Brey W., Gabbay E. J. Letter: Stereospecific interaction of dipeptide amides with DNA. Evidence for partial intercalation and bending of the helix. J Am Chem Soc. 1975 Apr 2;97(7):1959–1961. doi: 10.1021/ja00840a062. [DOI] [PubMed] [Google Scholar]
- Bernier J. L., Henichart J. P., Catteau J. P. Design, synthesis and DNA-binding capacity of a new peptidic bifunctional intercalating agent. Biochem J. 1981 Dec 1;199(3):479–484. doi: 10.1042/bj1990479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier J. L., Henichart J. P., Catteau J. P. ESR study of intercalation: quantitative evaluation of drug-DNA binding through competition with a spin-labeled 9-aminoacridine. Anal Biochem. 1981 Oct;117(1):12–17. doi: 10.1016/0003-2697(81)90683-7. [DOI] [PubMed] [Google Scholar]
- Booth T. E., Sakai T. T., Glickson J. D. Interaction of bleomycin A2 with poly(deoxyadenylylthymidylic acid). A proton nuclear magnetic resonance study of the influence of temperature, pH, and ionic strength. Biochemistry. 1983 Aug 30;22(18):4211–4217. doi: 10.1021/bi00287a008. [DOI] [PubMed] [Google Scholar]
- Chen D. M., Sakai T. T., Glickson J. D., Patel D. J. Bleomycin-A2 complexes with poly(dA--dT): a proton nuclear magnetic resonance study of the nonexchangeable hydrogens. Biochem Biophys Res Commun. 1980 Jan 15;92(1):197–205. doi: 10.1016/0006-291x(80)91539-9. [DOI] [PubMed] [Google Scholar]
- Cohen G., Eisenberg H. Conformation studies on the sodium and cesium salts of calf thymus deoxyribonucleic acid (DNA). Biopolymers. 1966 Apr-May;4(4):429–440. doi: 10.1002/bip.1966.360040404. [DOI] [PubMed] [Google Scholar]
- Dabrowiak J. C., Greenaway F. T., Grulich R. Transition-metal binding site of bleomycin A2. A carbon-13 nuclear magnetic resonance study of the zinc(II) and copper(II) derivatives. Biochemistry. 1978 Sep 19;17(19):4090–4096. doi: 10.1021/bi00612a034. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Adawadkar P. D., Kapicak L., Pearce S., Wilson W. D. The interaction specificity of peptides with DNA. Evidence for peptide beta-sheet-DNA binding. Biochemistry. 1976 Jan 13;15(1):152–157. doi: 10.1021/bi00646a023. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Adawadkar P. D., Wilson W. D. Stereospecific binding of diastereomeric peptides to salmon sperm DNA. Biochemistry. 1976 Jan 13;15(1):146–151. doi: 10.1021/bi00646a022. [DOI] [PubMed] [Google Scholar]
- Glickson J. D., Pillai R. P., Sakai T. T. Proton NMR studies of the Zn(II)--bleomycin-A2--poly(dA-dT) ternary complex. Proc Natl Acad Sci U S A. 1981 May;78(5):2967–2971. doi: 10.1073/pnas.78.5.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Piette L. H. Electron spin resonance spin-label studies of intercalation of ethidium bromide and aromatic amine carcinogens in DNA. Cancer Res. 1976 Mar;36(3):1159–1171. [PubMed] [Google Scholar]
- Hénichart J. P., Bernier J. L., Catteau J. P. Interaction of 4-(9-acridinylamino)aniline and derivatives with DNA. Influence of a lysylglycyl side chain on the binding parameters. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):835–841. doi: 10.1515/bchm2.1982.363.2.835. [DOI] [PubMed] [Google Scholar]
- Hénichart J. P., Bernier J. L., Houssin R., Lohez M., Kenani A., Catteau J. P. Copper(II)- and iron(II)-complexes of methyl 2-(2-aminoethyl)-aminomethyl-pyridine-6-carboxyl-histidinate (AMPHIS), a peptide mimicking the metal-chelating moiety of bleomycin. An ESR investigation. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1036–1041. doi: 10.1016/0006-291x(85)90289-x. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Lanier A. C., Keel R. A., Wilson W. D. The effect of ionic strength on DNA-ligand unwinding angles for acridine and quinoline derivatives. Nucleic Acids Res. 1980 Apr 11;8(7):1613–1624. doi: 10.1093/nar/8.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapicak L., Gabbay E. J. Effect of aromatic cations on the tertiary structure of deoxyribonucleic acid. J Am Chem Soc. 1975 Jan 22;97(2):403–408. doi: 10.1021/ja00835a031. [DOI] [PubMed] [Google Scholar]
- Krueger W. C., Pschigoda L. M., Reusser F. Interactions of DNA with zorbamycin, phleomycin and bleomycin; ultraviolet absorption and circular dichroism measurements. J Antibiot (Tokyo) 1973 Aug;26(8):424–428. doi: 10.7164/antibiotics.26.424. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., KLINE B., MEHROTRA B. D. SOME OBSERVATIONS ON THE HYPOCHROMISM OF DNA. J Mol Biol. 1964 Sep;9:801–811. doi: 10.1016/s0022-2836(64)80186-8. [DOI] [PubMed] [Google Scholar]
- Murakami H., Mori H., Taira S. On the molecular embrace of bleomycin with helical DNA. J Theor Biol. 1976 Jun;59(1):1–23. doi: 10.1016/s0022-5193(76)80021-5. [DOI] [PubMed] [Google Scholar]
- Nagai K., Yamaki H., Suzuki H., Tanaka N., Umezawa H. The combined effects of bleomycin and sulfhydryl compounds on the thermal denaturation of DNA. Biochim Biophys Acta. 1969 Mar 18;179(1):165–171. doi: 10.1016/0005-2787(69)90132-4. [DOI] [PubMed] [Google Scholar]
- Neidle S., Abraham Z. Structural and sequence-dependent aspects of drug intercalation into nucleic acids. CRC Crit Rev Biochem. 1984;17(1):73–121. doi: 10.3109/10409238409110270. [DOI] [PubMed] [Google Scholar]
- Oppenheimer N. J., Rodriguez L. O., Hecht S. M. Structural studies of of "active complex" of bleomycin: assignment of ligands to the ferrous ion in a ferrous-bleomycin-carbon monoxide complex. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5616–5620. doi: 10.1073/pnas.76.11.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Hogan M., Dattagupta N. Binding of bleomycin to DNA: intercalation of the bithiazole rings. Biochemistry. 1979 Jan 9;18(1):96–101. doi: 10.1021/bi00568a015. [DOI] [PubMed] [Google Scholar]
- Roy S. N., Orr G. A., Brewer C. F., Horwitz S. B. Chemical synthesis of radiolabeled bleomycin A2 and its binding to DNA. Cancer Res. 1981 Nov;41(11 Pt 1):4471–4477. [PubMed] [Google Scholar]
- Révet B. M., Schmir M., Vinograd J. Direct determination of the superhelix density of closed circular DNA by viscometric titration. Nat New Biol. 1971 Jan 6;229(1):10–13. doi: 10.1038/newbio229010a0. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Festy B., Le Pecq J. B. The change of the torsion of the DNA helix caused by intercalation. II. Measurement of the relative change of torsion induced by various intercalating drugs. Biochimie. 1971;53(9):973–980. doi: 10.1016/s0300-9084(71)80065-2. [DOI] [PubMed] [Google Scholar]
- Sausville E. A., Stein R. W., Peisach J., Horwitz S. B. Properties and products of the degradation of DNA by bleomycin and iron(II). Biochemistry. 1978 Jul 11;17(14):2746–2754. doi: 10.1021/bi00607a008. [DOI] [PubMed] [Google Scholar]
- Sheardy R. D., Gabbay E. J. Stereospecific binding of diastereomeric peptides to salmon sperm deoxyribonucleic acid. Further evidence for partial intercalation. Biochemistry. 1983 Apr 26;22(9):2061–2067. doi: 10.1021/bi00278a005. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Reddy B. S., Bhandary K. K., Jain S. C., Sakore T. D., Seshadri T. P. Conformational flexibility in DNA structure as revealed by structural studies of drug intercalation and its broader implications in understanding the organization of DNA in chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):87–102. doi: 10.1101/sqb.1978.042.01.010. [DOI] [PubMed] [Google Scholar]
- Wakelin L. P., Waring M. J. The unwinding of circular deoxyribonucleic acid by phenanthridinium drugs: structure-activity relations for the intercalation reaction. Mol Pharmacol. 1974 May;10(3):544–561. [PubMed] [Google Scholar]
- Wakelin S. P., Waring M. J. The binding of echinomycin to deoxyribonucleic acid. Biochem J. 1976 Sep 1;157(3):721–740. doi: 10.1042/bj1570721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]


