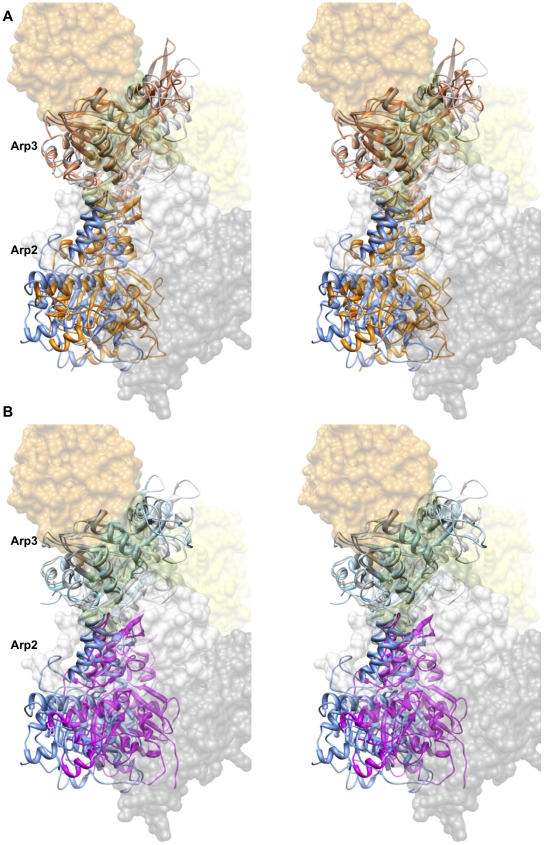
Figure 5. Structural changes induced by R105A ARPC4 mutants in the absence and presence of phosphorylation.
Stereoimages of changes in Arp2-Arp3 orientation in R105A ARPC4 mutant Arp2/3 complex from snapshots from the last ns of the unphosphorylated wild-type(Arp3-gray; Arp2-blue) and the (a) unphosphorylated R105A ARPC4 mutant complex (pink, orange) or (b) phosphorylated T237 Arp2 ARPC4 R105A ARPC4 mutant complex (cyan, magenta) simulations are shown following alignment of Cα atoms of subdomains 1 and 2 of Arp3. The other subunits of the complex from the snapshot of the unphosphorylated simulation, colored as in Fig. 2a, were represented as transparent surfaces in order not to occlude the views of Arp2 and Arp3.