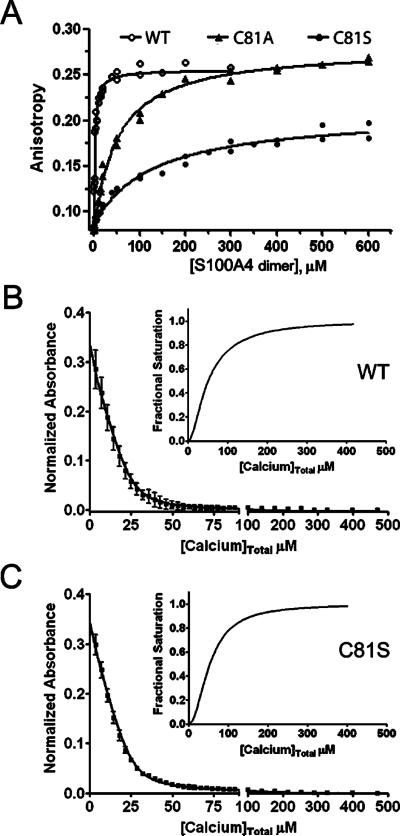
Figure 4.
Biochemical characterization of S100A4 C81 mutants. (A) Fluorescence anisotropy measurements of wild-type S100A4 or C81 mutants. Dissociation constants of 46.6 ± 3.2 and 128.4 ± 15.7 μM were obtained for C81A and C81S, respectively. Values represent the mean ± standard deviation from two independent experiments. Ca2+-binding to wild-type S100A4 (B) and C81S S100A4 (C). Dissociation constants for Ca2+-binding were determined using a competition assay with the chromophoric Ca2+ chelator 5,5′Br2-BAPTA. The data represent two independent experiments. The insets show the saturation curve representation for the best fit in CaLigator. Wild-type and C81S S100A4 bind Ca2+ at EF2 with comparable affnities of 5.8 ± 0.6 and 4.2 ± 0.2 μM, respectively.