Figure 3.
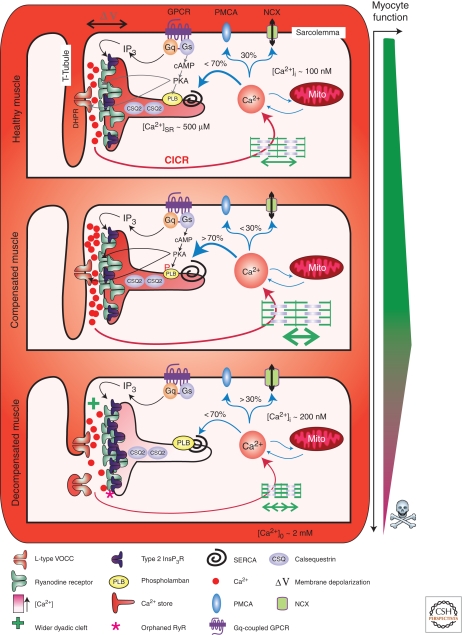
Excitation contraction coupling in healthy and decompensated hypertrophic cardiac muscle. The figure depicts the architecture and molecular composition of a cardiac dyad in a normal, healthy myocyte (upper panel), a compensated hypertrophic situation (middle panel), and a decompensated failing myocyte (lower panel). A key depicting the symbols used to represent the major players in EC-coupling is provided at the bottom of the figure. Depolarization of the plasma membrane results in Ca2+ influx through L-type voltage gated channels in the T-Tubule, which stimulates Ca2+ release via RyRs located on the juxtaposed SR. Following diffusion out of the dyadic cleft, Ca2+ encounters the contractile filaments causing myocyte contraction. Myocyte relaxation is then brought about by Ca2+ recycling back into the SR by the SERCA pump or extrusion across the plasma membrane via NCX. Neurohormonal activation of Gαq-coupled receptors leads to the formation of InsP3, which stimulates Ca2+ release via InsP3Rs located in the dyad. This InsP3-stimulated Ca2+ release sensitizes neighboring RyRs, causing enhanced Ca2+ fluxes and increasing the frequency of arrhythmic events. Activation of β-adrenergic receptors increases intracellular cAMP, which activates PKA leading to phosphorylation of PLB. On phosphorylation, PLB dissociates from SERCA thereby enhancing Ca2+ transport activity. Compensated/adaptive hypertrophy is associated with enhanced EC-coupling. Significantly contributing to this phenotype is an increase in SR store loading. This is brought about by an up-regulation of SERCA activity mediated by either an increase in SERCA or decrease in PLB expression. Alternatively, as a result of PKA-dependent phosphorylation, PLB interaction with SERCA and suppression of Ca2+ transport may also be decreased. Increased SERCA activity also serves to increase the rate of relaxation thereby allowing more rapid cycles of myocyte contraction. The width of the dyadic cleft may also marginally increase at this stage of hypertrophic remodeling. However, IP3Rs are up-regulated in the dyad during hypertrophy supplementing the Ca2+ signal arising via RyRs to possibly further support EC-coupling. During decompensated hypertrophy, myocyte architecture and protein expression are remodeled. Specifically, the width of the dyadic cleft is increased making it harder for Ca2+ arising via VOCCs to activate Ca2+ release from RyRs. T-Tubules also atrophy resulting in orphaned RyRs. The SERCA-PLB ratio is also modified to favor decreased SERCA activity. Notably, InsP3R expression in the dyad is increased. As a result, more of the RyRs that are located in this region are close enough to InsP3Rs to be affected by Ca2+ arising from them. Overactivation of these InsP3Rs, for example, by the elevated levels of circulating ET-1 present during heart failure, promotes arrhythmias thereby contributing to the pathology associated with heart failure.