Abstract
Damage-associated molecular pattern molecules such as high-mobility group box 1 protein (HMGB1) and heat shock protein 70 (HSP70) have been implicated in the pathogenesis of asthma. The aim of our study was to examine the induced sputum and plasma concentrations of HSP70 in asthmatic patients to determine their relationship with airway obstruction. Thirty-four healthy controls and 56 patients with persistent bronchial asthma matched for gender and age were enrolled in this study. Spirometry measurements were performed before sputum induction. HSP70 levels in induced sputum and plasma were measured using the ELISA Kit. Sputum and plasma concentrations of HSP70 in asthmatics patients were significantly higher than that in control subjects (sputum, (0.88 ng/ml (0.27–1.88 ng/ml) versus 0.42 ng/ml (0.18–0.85 ng/ml), p < 0.001); plasma, (0.46 ng/ml (0.20–0.98 ng/ml) versus 0.14 ng/ml (0.11–0.37 ng/ml), p < 0.001) and were significantly negatively correlated with forced expiratory volume in 1 s (FEV1), FEV1 (percent predicted), and FEV1/FVC in all 90 participants and 56 patients with asthma. There were no significant differences in HSP70 levels between patients with eosinophilic and non-eosinophilic asthma. HSP70 levels in plasma were positively correlated with neutrophil count, and HSP70 levels in induced sputum were positively correlated with lymphocyte count. In multivariate analysis, independent predictors of sputum HSP70 were diseases and disease severity but not smoking, age, or gender, and independent predictors of plasma HSP70 were also diseases and disease severity. In conclusion, this study indicates that induced sputum and plasma HSP70 could serve as a useful marker for assessing the degree of airway obstruction in patients with asthma. However, further investigation is needed to establish the role of circulating and sputum HSP70 in the pathogenesis of asthma.
Keywords: Asthma, Heat shock protein 70, Induced sputum, Lung functions, Damage-associated molecular patterns
Inflammatory mediators play important roles in the development of chronic inflammation like asthma. Recent studies have demonstrated that apoptotic and necrotic cells release damage-associated molecular pattern (DAMP) molecules such as high-mobility group box-1 (HMGB1), heat shock proteins (HSPs), and S-100 proteins, which promote early innate and adaptive immune responses through their interaction with pattern recognition receptors (Kono and Rock 2008; Srikrishna and Freeze 2009). DAMPs contribute to the induction of inflammation by activation and recruitment of various inflammatory cells. Studies have suggested an important role of DAMPs in the pathogenesis of allergic disease (Willart and Lambrecht 2009). Recently, we have reported that the HMGB1 level, one of the important DAMPs, is increased in patients with asthma and chronic obstructive pulmonary disease (COPD) (Hou et al. 2011) and significantly negatively correlated with pulmonary function index. We also reported that HSP might be involved in the pathogenesis of asthma (Tong and Luo 2000).Study also showed that S100A8/A9 may maintain and strengthen the chronic airway inflammation and airway remodeling in asthma by inducing effector responses of resident and infiltrating airway cells (Halayko and Ghavami 2009).
However, it is still unknown how HSP70 (also named HSPA1A, HSP70 refers to this protein later in the manuscript) (Kampinga et al. 2009; Hageman and Kampinga 2009), another one of DAMPs, was involved in the pathophysiology of asthma. Heat shock or stress proteins (HSPs) are a family of highly conserved proteins found in cells of all organisms, from bacteria to mammals. Of all heat shock proteins, the HSP70 family constitutes the most conserved and best-studied class. Intracellularly, HSPs function to stabilize the cell and maintain cellular homeostasis (Hartl 1996). However, during the response to stress or injury like temperature, exercise, and infection, HSP-70 may be released from dying cells that have lysed, as well as from live cells via receptor-mediated exocytosis (Asea 2007; Clayton et al. 2005; Hunter-Lavin et al. 2004). When HSPs are released into the extracellular compartment, they are able to deliver a partial maturation signal to dendritic cells, which may play a crucial role in pathogenesis of asthma and activate the NF-kappaB pathway, and can also stimulate pro-inflammatory cytokines production in vitro (Asea et al. 2000; Basu et al. 2000). This suggests that HSP70 may modulate and exert immune and inflammatory responses and may be involved in the pathogenesis and/or be markers of some diseases like atherosclerosis, ischemia–reperfusion injury, preeclampsia, and HELLP syndrome (Bielecka-Dabrowa et al. 2009; Molvarec et al. 2007, 2009, 2010; Walsh et al. 2009). Meanwhile, it has been found that extracellular HSP70 level is increased in patients with sarcoidosis and chronic beryllium disease (Silva et al. 2007) and COPD patients (Hacker et al. 2009). In Yang’s study, the presence of autoantibodies against Hsp70 was associated with the severity of asthma and was also correlated with higher levels of total IgE and IL-4 in asthmatic patients (Yang et al. 2005). Tamási et al. (2010) have recently reported that the serum HSP70 levels increased in asthmatic pregnant women compared to the control healthy pregnant and the levels of serum HSP70 negatively correlated with the maternal age and positively correlated with gestational age in the group of healthy pregnant women. These data suggest that the HSP70 may be involved in the pathogenesis and development of these diseases including asthma. However, the induced sputum and plasma concentrations of HSP70 have not been systematically evaluated in patients with asthma.
We hypothesized that HSP70 expression is increased in asthma and is related to disease severity. To test our hypothesis, we have measured HSP70 concentrations in induced sputum and plasma of control subjects, patients with untreated asthma.
Materials and methods
Subjects
We selected 56 asthmatic patients from patients at the Department of Respiratory Medicine of Southern Medical University Nanfang Hospital (Guangzhou, China). Asthmatic patients in our study were firstly diagnosed in our hospital according to the Global Initiative for Asthma guidelines (Bateman et al. 2008). Classification of asthma severity was based on symptom and forced expiratory volume in 1 s (FEV1) (Bateman et al. 2008). For mild asthma, FEV1 ≥ 80% predicted and asthma symptoms more than once but less than once a day or nocturnal symptoms more than twice a month; for moderate asthma, FEV1 = 60%–80% predicted and daily symptoms or nocturnal symptoms more than once a week; and for severe asthma, FEV1 ≤ 60% predicted and symptoms daily or frequent nocturnal asthma symptoms and limitation of physical activities. Inclusion criteria included patients or participants who had not taken corticosteroids (oral or injected), nonsteroidal anti-inflammatory medications (cromolyn, ketotifen, leukotriene receptor antagonists), long-acting beta-2-agonists, or aminophylline 3 months prior to this study. Exclusion criteria included (1) respiratory tract infection characterized by purulent sputum and infiltration based on X-ray or computed tomography scans of the lungs within the previous 6 weeks, (2) history of any other lung disease except asthma, and (3) other diseases with increased levels of HSP70 (atherosclerosis, multiple sclerosis, COPD). Thirty-four healthy volunteers as the control subjects included those without asthma or COPD, pneumonia. The study was approved by the ethics committee of Southern Medical University, and all subjects and patients provided informed consent for participation.
Pulmonary function tests
Spirometry measurements were performed before sputum induction. Spirometry was performed using the Jaeger Masterscope® spirometry system (Jaeger, Wuerzburg, Germany) according to American Thoracic Society (ATS) guidelines (Miller et al. 2005).
Asthma patients’ symptom score
Asthma patients were instructed to record daytime symptoms of cough, chest tightness, wheeze, sputum production, breathlessness, grading each from 0–3 (no symptoms = 0, mild = 1, moderate = 2, and severe = 3) (Santanello et al. 1997) and nocturnal symptoms for the preceding 24 h, how many times they woke up with asthma symptoms, grading each from 0–3 (none = 0, once = 1, more than once = 2, awake all night = 3) (Fletcher et al. 1959).
Blood sample preparation
Blood was drawn, handled, and stored by the same researcher in the same department. Blood samples were collected in ethylenediamine tetraacetic acid-anticoagulated PB tubes and stored at 4°C. Blood samples were centrifuged at 1,000×g at 4°C for 15 min and were stored in microfuge tubes at −80°C until the measurements were taken.
Sputum induction and processing
For the sputum induction and processing, we used the guidelines suggested by the Task Force on Induced Sputum of the European Respiratory Society (Paggiaro et al. 2002).Subjects and patients were told to periodically spit saliva into one container and sputum into another. The sputum was weighed and diluted four times in fresh 0.1% dithiothreitol (Sigma-Aldrich; St Louis, USA) in distilled water. The suspension was shaken in a vortex mixer for a few seconds and incubated in a shaking water bath at 37°C (150 cycles/min) for 15 min with aspiration every 5 min for homogenization. Samples were centrifuged at 750×g for 10 min. The supernatant was aspirated and stored, and total cell number and cell viability were determined by the trypan blue dye exclusion method in a Neubauer chamber. Slides were prepared for differential cell counts by cytospin staining with hematoxylin–eosin.
HSP70 measurement
Plasma and sputum HSP70 were quantified using commercial HSP70 ELISA kit (Hyperheal, Shanghai, China). The detection limit of these kits was 0.05 ng/mL for HSP70. Each sample was run in duplicate and compared with a standard curve. The mean concentration was determined for each sample.
Statistical analysis
Results are expressed as median (range) unless otherwise specified. The normality of continuous variables was assessed using the Shapiro–Wilk’s W test. As the continuous variables were not normally distributed, nonparametric statistical were performed. Significant variation in the data among multiple groups was investigated using Kruskal–Wallis test. The Mann–Whitney U test (with Bonferroni’s correction) was used to compare difference between two groups. Frequency data (e.g., sex) were analyzed by the Chi-square test. Correlations were assessed by spearman rank correlation coefficients. A receiver operating characteristic (ROC) curve with its area under the curve was computed to evaluate the diagnostic accuracy of plasma and induced sputum HSP70 measurements. Multiple regressions analysis was performed for the evaluation of HSP70 predictors, using HSP70 in plasma or induced sputum as dependent variable and sex, age, smoking pack-years, control/asthma, and FEV1% predicted as independent variables. P values <0.05 were considered statistically significant.
Results
Clinical data
Demographic characteristics of the patients with asthma and control subjects are shown in Table 1. All patients and subjects were matched for gender, age, BMI, and smoking pack-years. The number of patients with a family history of asthma (16 of 56 patients) and allergic rhinitis (17of 56 patients) in asthma patient group was higher compared with those of healthy controls (4 of 34 participants and 3 of 34 participants, respectively). There was no difference in the total number of cells in induced sputum (not including the squamous cell) and peripheral white blood cells between patients with asthma and health controls. Asthma patients had higher percentage of sputum and blood eosinophils and lower percentage of sputum macrophages compared with healthy controls (p < 0.05). While asthma patients had higher percentage of sputum lymphocytes compared with healthy controls (p < 0.05).
Table 1.
Patient and subject demographics
| Characteristic | Control subjects | Asthma patients | P value |
|---|---|---|---|
| N | 34 | 56 | |
| Gender, male/female | 22/12 | 30/26 | 0.140 |
| Age, years | 39 (24–70) | 39 (18–62) | 0.733 |
| BMI | 22.13 ± 4.35 | 21.80 ± 2.77 | 0.873 |
| Never/current/ex-smokers | 29/5/0 | 41/6/9 | |
| Smoking history, pack-years | 0 (0–30) | 0 (0–30) | 0.760 |
| Induced sputum | |||
| TCCa (106/ml) | 3.55 (1.62–6.60) | 4.20 (1.74–11.36) | 0.141 |
| Macrophages, % | 52.5 (31.0–80.0) | 42 (8.0–90.50)* | 0.012 |
| Neutrophils, % | 43.75 (20.0–65.0) | 37.5 (1.0–70.0) | 0.061 |
| Lymphocytes, % | 2.0 (0.0–12.0) | 3.5 (0.0–24.0)* | 0.044 |
| Eosinophils, % | 1.0 (0.0–8.0) | 7.50 (0.0–83.0)* | 0.000 |
| FVC(L) | 3.51 (1.72–5.82) | 2.85 (1.36–4.57)* | 0.000 |
| PEF(L/s) | 7.97 (4.72-12.10) | 4.99 (1.42-10.75)* | 0.000 |
| FEV1(L) | 3.07 (2.00-4.14) | 2.14 (0.86-4.09)* | 0.000 |
| FEV1, % predicted | 102.70 (88.00–121.90) | 74.45 (24.40–116.30)* | 0.000 |
| FEV1/FVC | 92.0 (78.07–111.2) | 81.02 (44.99–100) | 0.000 |
| Daily score | 6 (1–12) | ||
| Nighttime score | 1 (0–3) | ||
| Peripheral blood counts | |||
| TCCb (109/ml) | 7.31 (5.58–8.21) | 6.74 (4.41–16.38) | 0.859 |
| Lymphocytes, % | 35.60 (28.20–42.80) | 29.55 (6.80–53.20) | 0.292 |
| Neutrophils, % | 56.30 (48.20–60.90) | 59.95 (35.10–85.40) | 0.943 |
| Macrophages, % | 6.95 (4.70–7.20) | 6.00 (1.25–17.20) | 0.519 |
| Eosinophils, % | 2.10 (1.40–2.90) | 5.20 (0.00–9.40)* | 0.045 |
Data are presented as median (range), except BMI is expressed as means ± SEM
BMI body mass index, TCC total cell count, FVC forced vital capacity, PEF peak expiratory flow, FEV1 forced expiratory volume in 1 s
*P < 0.05 versus control group
aTotal number of cells in induced sputum (not include the squamous cell
bTotal number of peripheral white blood cells
The different levels of HSP70 in induced sputum and plasma
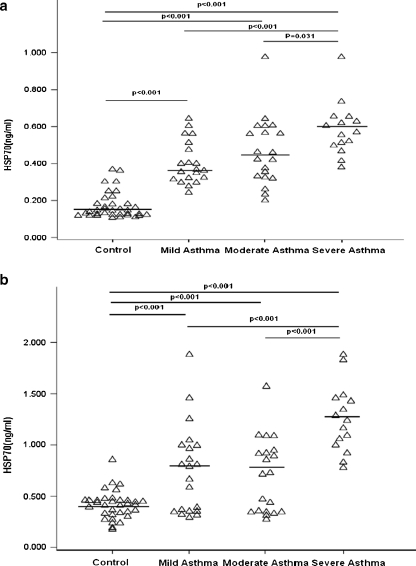
Differences in HSP70 between all subgroups of controls and asthma patients are presented in Fig. 1. Compared with controls (0.42 ng/ml (0.18–0.85 ng/ml)), in patients with all severities of asthma (0.88 ng/ml (0.27–1.88 ng/ml)), HSP70 in induced sputum was significantly increased (p < 0.001). Compared with controls (0.14 ng/ml (0.11–0.37 ng/ml)), in patients with all severities of asthma (0.46 ng/ml (0.20–0.98 ng/ml)), HSP70 in plasma was also significantly increased (p < 0.001). Similarly, compared with controls (0.14 ng/ml (0.11–0.37 ng/ml)), HSP70 in plasma was significantly higher in patients with mild asthma(0.36 ng/ml (0.24–0.64 ng/ml); n = 21; p < 0.001), in patients with moderate asthma(0.44 ng/ml (0.20–0.98 ng/ml); n = 20; p < 0.001) and severe asthma (0.57 ng/ml (0.38–0.98 ng/ml); n = 15; p < 0.001) (Mann–Whitney U test with Bonferroni’s correction).The plasma and sputum HSP70 level significantly increased in patients with severe asthma compared with mild asthma (p < 0.001; p < 0.001). The HSP70 levels in plasma were significantly higher in patients with severe asthma than those with moderate asthma (p = 0.031). Similarly, the HSP70 level in induced sputum was higher in patients with severe asthma (1.24 ng/ml (0.78–1.88 ng/ml)) compared with moderate asthma (0.72 ng/ml (0.27–1.68 ng/ml); p < 0.001) (Fig. 1b).There was no difference in plasma and induced sputum between patients with mild asthma and moderate asthma (p = 1.000; p = 1.000).
Fig. 1.
a HSP70 concentration in plasma from healthy control and asthma patients of different severity. b HSP70 concentration in induced sputum from healthy control, asthma patients of different severity. Kruskal–Wallis test (among multiple groups) and Mann–Whitney U test (between groups; with Bonferroni’s correction) were used. The line represents the median
Although non-eosinophilic asthma patients had higher HSP70 level in plasma and sputum compared with eosinophilic asthma patients’ plasma and sputum, differences were not significant (p = 0.573; p = 0.717, respectively). (Table 2).A cutoff point of 3% eosinophils in induced sputum was applied to distinguish eosinophilic from non-eosinophilic asthma patients (Simpson et al. 2010).
Table 2.
The HSP70 levels in plasma and induced sputum of healthy subjects, non-eosinophilic asthma patients and eosinophilic asthma patients
| Group | Number | Plasma (ng/ml) | Induced sputum (ng/ml) |
|---|---|---|---|
| Control | 34 | 0.14 (0.11–0.37) | 0.42 (0.18–0.85) |
| Non-eosinophilic asthma | 19 | 0.56 (0.23–0.98)* | 0.95 (0.34–1.88)* |
| Eosinophilic asthma | 37 | 0.42 (0.20–0.65)* | 0.81 (0.27–1.88)* |
| P value | <0.001 | <0.001 |
Results are expressed as median (range)
*P < 0.001vs control group
Using the ROC curve analysis, we determined a cutoff value of HSP70 concentration (0.65 ng/ml) in induced sputum, which can discriminate asthmatics from healthy control with 67.9% sensitivity and 97.1% specificity and a cutoff value of HSP70 concentration (0.26 ng/ml) in plasma, which can discriminate asthmatics from healthy control with 94.6% sensitivity and 91.2% specificity.
Correlation of HSP70 levels with lung function or other parameters
HSP70 levels in plasma and induced sputum in all subjects and patients showed significant negative correlation with lung function parameters such as FEV1, FEV1 (percent predicted) and FEV1/FVC. In all participants, plasma HSP70 levels showed stronger negative correlation with pulmonary function index than HSP70 levels in induced sputum.
HSP70 levels in all groups and the plasma from the asthma group were positively correlated with neutrophil count and its percentage. HSP70 levels in all groups and the induced sputum from the asthma group were positively correlated with lymphocyte count and its percentage. Although HSP70 levels in induced sputum were positively correlated with eosinophil count, no significant correlation was found in the asthma group.
In addition, in the asthma group, HSP70 levels in plasma correlated positively with the nighttime score, but not with daily score; in contrast, HSP70 levels in induced sputum correlated positively with the daily score, but not with nighttime score, in the asthma group (Tables 3 and 4).
Table 3.
Correlations between HSP70 concentration and lung function and cells in induced sputum and peripheral blood in all study subjects
| Including all subjects (n = 90) | |||||||
|---|---|---|---|---|---|---|---|
| Index | rs | P value | Index | rs | P value | ||
| P-HSP70 | FVC | −0.570 | 0.000 | S-HSP70 | FVC | −0.331 | 0.001 |
| P-HSP70 | PEF | −0.659 | 0.000 | S-HSP70 | PEF | −0.366 | 0.000 |
| P-HSP70 | FEV1 | −0.667 | 0.000 | S-HSP70 | FEV1 | −0.400 | 0.000 |
| P-HSP70 | FEV1% pred | −0.578 | 0.000 | S-HSP70 | FEV1% pred | −0.433 | 0.000 |
| P-HSP70 | FEV1/FVC | −0.483 | 0.000 | S-HSP70 | FEV1/FVC | −0.436 | 0.000 |
| P-HSP70 | B-neu% | 0.327 | 0.011 | S-HSP70 | S-neu% | 0.008 | 0.937 |
| P-HSP70 | B-neu | 0.251 | 0.049 | S-HSP70 | S-neu | 0.033 | 0.757 |
| P-HSP70 | B-eos | 0.008 | 0.953 | S-HSP70 | S-eos | 0.282 | 0.007 |
| P-HSP70 | B-lym% | −0.192 | 0.141 | S-HSP70 | S-lym% | 0.308 | 0.003 |
| P-HSP70 | B-lym | −0.139 | 0.289 | S-HSP70 | S-lym | 0.312 | 0.003 |
| P-HSP70 | S-HSP70 | 0.549 | 0.000 | ||||
Data are presented as Spearman rank correlation coefficients
P-HSP70 the HSP70 level in plasma, S-HSP70 the HSP70 level in sputum, FVC forced vital capacity, PEF peak expiratory flow, FEV1 forced expiratory volume in 1 s, B-neu neutrophils in peripheral blood, B-eos eosinophils in peripheral blood, B-lym lymphocyte in peripheral blood, S-neu neutrophils in induced sputum, S-eos eosinophils in induced sputum, S-lym lymphocyte in induced sputum
Table 4.
Correlations between HSP70 concentration and lung function, cells in induced sputum and peripheral blood and symptom score in asthmatic patients
| Asthma patients (n = 56) | |||||||
|---|---|---|---|---|---|---|---|
| Index | rs | P value | Index | rs | P value | ||
| P-HSP70 | FVC | −0.472 | 0.000 | S-HSP70 | FVC | −0.409 | 0.002 |
| P-HSP70 | PEF | −0.500 | 0.000 | S-HSP70 | PEF | −0.406 | 0.002 |
| P-HSP70 | FEV1 | −0.479 | 0.000 | S-HSP70 | FEV1 | −0.455 | 0.000 |
| P-HSP70 | FEV1% pred | −0.335 | 0.012 | S-HSP70 | FEV1% pred | −0.437 | 0.000 |
| P-HSP70 | FEV1/FVC | −0.356 | 0.008 | S-HSP70 | FEV1/FVC | −0.332 | 0.011 |
| P-HSP70 | B-neu% | 0.349 | 0.009 | S-HSP70 | S-neu% | 0.136 | 0.319 |
| P-HSP70 | B-neu | 0.321 | 0.016 | S-HSP70 | S-neu | 0.090 | 0.509 |
| P-HSP70 | B-eos | −0.106 | 0.438 | S-HSP70 | S-eos | −0.071 | 0.601 |
| P-HSP70 | B-lym% | −0.154 | 0.258 | S-HSP70 | S-lym% | 0.278 | 0.038 |
| P-HSP70 | B-lym | −0.070 | 0.606 | S-HSP70 | S-lym | 0.266 | 0.048 |
| P-HSP70 | Daily score | 0.152 | 0.263 | S-HSP70 | Daily score | 0.421 | 0.001 |
| P-HSP70 | Night time score | 0.356 | 0.007 | S-HSP70 | Night time score | 0.166 | 0.223 |
| P-HSP70 | S-HSP70 | 0.497 | 0.000 | ||||
Data are presented as Spearman rank correlation coefficients
P-HSP70 the HSP70 level in plasma, S-HSP70 the HSP70 level in sputum, FVC forced vital capacity, PEF peak expiratory flow, FEV1 forced expiratory volume in 1 s, B-neu neutrophils in peripheral blood, B-eos eosinophils in peripheral blood, B-lym lymphocyte in peripheral blood, S-neu neutrophils in induced sputum, S-eos eosinophils in induced sputum, S-lym lymphocyte in induced sputum
Evaluation of HSP70 predictors with multiple regressions analysis
Predictors of HSP70 in plasma, provided by multiple regression analysis, are presented in Table 5. Disease severity and control/asthma but not sex, age, and smoking pack-years were independent predictors of HSP70 (R2 = 0.639, adjusted R2 = 0.617). And predictors of HSP70 in sputum were also disease severity and control/asthma (R2 = 0.404, adjusted R2 = 0.368) (Table 6).
Tables 5.
Parameters associated with HSP70 level in plasma
| Variables | Unstandardized coefficients(95% CI), | Standardized coefficients | P value |
|---|---|---|---|
| Gender | 32.26 (3.15∼61.37) | 0.079 | 0.271 |
| Age | 3.653 (2.434∼4.872) | 0.101 | 0.104 |
| Smoking pack-years | −0.386 (−2.286∼1.514) | −0.015 | 0.840 |
| Control/asthma | 242.55 (208.54∼276.56) | 0.585 | 0.000 |
| FEV1, %predicted | −2.040 (−2.724∼−1.356) | −0.246 | 0.004 |
| Constant | −59.380 (−171.27∼52.51) | 0.597 |
Tables 6.
Parameters associated with HSP70 level in induced sputum
| Variables | Unstandardized coefficients (95% CI) | Standardized coefficients | P value |
|---|---|---|---|
| Gender | 102.81 (24.94∼180.68) | 0.121 | 0.190 |
| Age | 4.535 (1.274∼7.796) | 0.120 | 0.168 |
| Smoking pack-years | 0.517 (−4.595∼5.629) | 0.009 | 0.920 |
| Control/asthma | 239.88 (148.89∼330.87) | 0.278 | 0.010 |
| FEV1, %predicted | −6.901 (−8.732∼−7.07) | −0.400 | 0.000 |
| Constant | 542.99 (243.65∼842.33) | 0.073 |
Discussion
We report here for the first time that the sputum HSP70 concentrations in asthma and plasma HSP70 concentrations in asthma without pregnancy were increased, dependent of disease severity. We also show that the concentrations of HSP70 positively and significantly correlated with neutrophil counts and percentage of neutrophils in peripheral blood and lymphocyte count and its percentage in induced sputum. Finally, consistent with a recent findings by Tamási et al. (2010), our reports confirmed that HSP70 concentrations are elevated in asthma patients and positively and significantly correlated with the asthma symptom scores.
As is well known, a wide variety of stressful stimulus including some drugs like estrogen (Stice and Knowlton 2008) can affect the expression and levels of HSPs, so in this study, we enrolled asthmatic patients who were firstly diagnosed in our hospital and had not taken corticosteroids, nonsteroidal anti-inflammatory medications, long-acting beta-2-agonists, or aminophylline 3 months prior to this study. Compared with healthy control, HSP70 levels in induced sputum were significantly increased in asthmatic patients, even after proper adjustments of gender, age, BMI, and smoking pack-years (data were not presented). The fact that disease severity was an independent predictor of plasma and sputum, HSP70 further supports a potential role for HSP70 as a new biomarker for asthma.
Tamási et al. (2010) reported a significant increase in the serum levels of HSP70 in asthmatic women during gestation. And Yang et al. (2005)demonstrated a significant increase of autoantibodies against HSP70 in patients with asthma. Also, elevated serum levels of HSP70 have recently been demonstrated in patients with COPD. However, to the best our knowledge, no studies have been performed yet to evaluate the sputum HSP70 levels in patients with asthma. And comparing results of the present study to those of Tamási et al. (2010), they investigated pregnant women.
It has been demonstrated that induced sputum is more concentrated and richer in airway secretions than BAL samples (Fahy et al. 1995), and samples from induced sputum are valid in assessing conditions involving airway inflammation, and sputum testing is safe and noninvasive. Although our results need further clarification, they indicate that HSP70 in induced sputum may be a novel and useful marker for reflecting disease severity of asthma.
Non-eosinophilic and eosinophilic asthma are likely produced by different immunological mechanisms, including a difference in cytokine production (Quaedvlieg et al. 2006). Accordingly, we compared the concentration of HSP70 in these two phenotypes. However, in this study, we did not find significant differences between non-eosinophilic asthma and eosinophilic asthma patients, a finding that needs further investigation. One reason for this may be that there were different sources of HSP70 such as from mast cells, lymphocyte, and neutrophils (Asea 2007; Clayton et al. 2005; Hunter-Lavin et al. 2004).
Our finding that HSP70 in plasma correlated positively with neutrophil count and its percentage in all participants and in asthma group is also supported by the findings that neutrophils contribute to increased levels of HSP70 (Giraldo et al. 2010; Persson et al. 2008), and extracellular Hsp72 released from virally infected airway epithelial cells can result in the recruitment and activation of neutrophils (Vignola et al. 1995; Wheeler et al. 2009).These findings suggest that neutrophils may be an important source of HSP70, and there may exist a positive feedback loop between HSP70 and neutrophils, an area we intend to explore in future studies. It was reported that HSP70 has been shown to be released by B cells which is consistent with our finding that the count and percentage of lymphocyte correlated positively with HSP70 levels in induced sputum from asthma patients (Clayton et al. 2005). One study suggests the mast cells may also be a potential source of HSP70 (Mortaz et al. 2006).
Understanding the role of HSP70 in asthma is complex, and much remains to be clarified. HSP70 has been suggested to play a role in asthma (Bertorelli et al. 1998; Willart and Lambrecht 2009). Airway cells (epithelial cells and alveolar macrophages) as well as peripheral blood mononuclear cells showed increased expression of HSP70 in asthma (Bertorelli et al. 1998; Tong and Luo 2000). HSP70 displays a wide range of immunological effects in addition to its cytokine effects, and it can be considered an alarm that alerts our defense system of an impending danger. To better describe the unique function as both chaperone and cytokine, extracellular HSP70 (eHSP70) was named as chaperokine by Asea (2003, 2005). eHSP70 can induce the antigen presenting cells (APC) maturation by augmenting the surface expression of CD40, CD83, CD86, and MHC class II molecules on APC and migration of APC (Asea 2005; Asea et al. 2002; Srivastava 2002); on the other hand, after exposure of APC to exogenous eHSP70, there is significant release of cytokines including TNF-α, IL-1β, IL-6, and IL-12 (Srivastava 2002), and chemokines including MIP-1, MCP-1, and RANTES. In addition, eHSP70 has been shown to stimulate monocytes and macrophages to produce pro-inflammatory cytokines such as TNF-, IL-6, and IL-12p40 (Rico et al. 1999) and to stimulate the proliferation of T cell and proliferation on B cell populations (Rico et al. 1999, 2002). These data and our results support the hypothesis that the HSP70 over-expression may play a potential and important role in the initiation and maintenance of chronic airway inflammation in asthma.
However, many studies also showed that HSP70 had an anti-inflammatory role in various inflammatory conditions such as infection, ischemia/reperfusion injury, and cardiovascular diseases (Bielecka-Dabrowa et al. 2009; Chen et al. 2007). This special phenomenon was described by Chen et al. (2007) as “heat shock paradox.” Rha et al. (2002) have reported that administration of Mycobacterium leprae HSP prevented both development of airway hyperreactivity (AHR) as well as bronchoalveolar lavage fluid (BALF) eosinophilia in a dose-dependent manner and the production of IL-4 and IL-5 in BALF. Although it is still unknown how to explain this phenomenon, some researches suggest that some factors play a crucial role in the effect of HSP. First of all, it is the time; HSPs induction before a pro-inflammatory stimulus is clearly beneficial; however, HSPs induction after a pro-inflammatory stimulus is cytotoxic. The second factor is place and sources; HSPs from extracellular or intracellular and different microorganisms may have different role (Galloway et al. 2008; Chen et al. 2007; Rha et al. 2002). Nevertheless, our results definitely support HSP70 as a new biomarker for evaluating the degree of airway obstruction in asthma; however, the source of HSPs in healthy individuals, as well as in patients with pathological conditions, and its exact mechanisms in allergic asthma have not been completely determined yet.
In conclusion, our finding that increased induced sputum and circulating HSP70 levels in patients with asthma are associated with disease severity and asthmatic symptom’ scores supports the hypothesis that HSP70 may play an important role in the pathogenesis of asthma and HSP70 may be an attractive therapeutic target for asthma. Also, plasma HSP70 may be a diagnostic tool for diagnosis of asthma. Further investigation will be necessary to determinate the sites, mechanisms, and source of HSP70 in asthma.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30971328), Doctoral Fund of Ministry of Education of China (20094433110011), and the Chronic Respiratory Disease Research Projects of Chinese Medical Association (no. 07010130021).
Footnotes
Haijin Zhao and Changchun Hou made equal contributions to this paper.
An erratum to this article can be found at http://dx.doi.org/10.1007/s12192-011-0278-2
Contributor Information
Zhao Haijin, Email: haijin99@sina.com.cn.
Cai Shao-xi, Phone: +86-20-61641571, Email: caishaox@fimmu.com.
Zou Fei, Email: zfei@fimmu.com.
References
- Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- Bertorelli G, Bocchino V, Zhuo X, Chetta A, Del DM, Foresi A, Testi R, Olivieri D. Heat shock protein 70 upregulation is related to HLA-DR expression in bronchial asthma. Effects of inhaled glucocorticoids. Clin Exp Allergy. 1998;28:551–560. doi: 10.1046/j.1365-2222.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- Bielecka-Dabrowa A, Barylski M, Mikhailidis DP, Rysz J, Banach M. HSP 70 and atherosclerosis—protector or activator? Expert Opin Ther Targets. 2009;13:307–317. doi: 10.1517/14728220902725149. [DOI] [PubMed] [Google Scholar]
- Chen Y, Voegeli TS, Liu PP, Noble EG, Currie RW. Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflamm Allergy Drug Targets. 2007;6:91–100. doi: 10.2174/187152807780832274. [DOI] [PubMed] [Google Scholar]
- Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Wong H, Liu J, Boushey HA. Comparison of samples collected by sputum induction and bronchoscopy from asthmatic and healthy subjects. Am J Respir Crit Care Med. 1995;152:53–58. doi: 10.1164/ajrccm.152.1.7599862. [DOI] [PubMed] [Google Scholar]
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2:257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway E, Shin T, Huber N, Eismann T, Kuboki S, Schuster R, Blanchard J, Wong HR, Lentsch AB. Activation of hepatocytes by extracellular heat shock protein 72. Am J Physiol Cell Physiol. 2008;295:C514–C520. doi: 10.1152/ajpcell.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo E, Multhoff G, Ortega E. Noradrenaline increases the expression and release of Hsp72 by human neutrophils. Brain Behav Immun. 2010;24:672–677. doi: 10.1016/j.bbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Hacker S, Lambers C, Hoetzenecker K, Pollreisz A, Aigner C, Lichtenauer M, Mangold A, Niederpold T, Zimmermann M, Taghavi S, Klepetko W, Ankersmit HJ. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55:31–40. [PubMed] [Google Scholar]
- Hageman J, Kampinga HH. Computational analysis of the human HSPH/HSPA/DNAJ family and cloning of a human HSPH/HSPA/DNAJ expression library. Cell Stress Chaperones. 2009;14:1–21. doi: 10.1007/s12192-008-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halayko AJ, Ghavami S. S100A8/A9: a mediator of severe asthma pathogenesis and morbidity? Can J Physiol Pharmacol. 2009;87:743–755. doi: 10.1139/Y09-054. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y , Shen X, Liang Z, Cai S, Zou F (2011) HMGB1 in Asthma: comparison with COPD and health controls. Mol Med. Mar 3. doi:10.2119/molmed.2010.00173. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Prohászka Z, Nagy B, Kalabay L, Szalay J, Füst G, Karádi I, Rigó J., Jr Association of increased serum heat shock protein 70 and C-reactive protein concentrations and decreased serum alpha(2)-HS glycoprotein concentration with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. J Reprod Immunol. 2007;73:172–179. doi: 10.1016/j.jri.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Rigó J, Jr, Lázár L, Balogh K, Makó V, Cervenak L, Mézes M, Prohászka Z. Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones. 2009;14:151–159. doi: 10.1007/s12192-008-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvarec A, Tamási L, Losonczy G, Madách K, Prohászka Z, Rigó J., Jr Circulating heat shock protein 70 (HSPA1A) in normal and pathological pregnancies. Cell Stress Chaperones. 2010;15:237–247. doi: 10.1007/s12192-009-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E, Redegeld FA, Nijkamp FP, Wong HR, Engels F. Acetylsalicylic acid-induced release of HSP70 from mast cells results in cell activation through TLR pathway. Exp Hematol. 2006;34:8–18. doi: 10.1016/j.exphem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Paggiaro PL, Chanez P, Holz O, Ind PW, Djukanovic R, Maestrelli P, Sterk PJ. Sputum induction. Eur Respir J Suppl. 2002;37:3s–8s. doi: 10.1183/09031936.02.00000302. [DOI] [PubMed] [Google Scholar]
- Persson YA, Blomgran-Julinder R, Rahman S, Zheng L, Stendahl O. Mycobacterium tuberculosis-induced apoptotic neutrophils trigger a pro-inflammatory response in macrophages through release of heat shock protein 72, acting in synergy with the bacteria. Microbes Infect. 2008;10:233–240. doi: 10.1016/j.micinf.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Quaedvlieg V, Henket M, Sele J, Louis R. Cytokine production from sputum cells in eosinophilic versus non-eosinophilic asthmatics. Clin Exp Immunol. 2006;143:161–166. doi: 10.1111/j.1365-2249.2005.02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha YH, Taube C, Haczku A, Joetham A, Takeda K, Duez C, Siegel M, Aydintug MK, Born WK, Dakhama A, Gelfand EW. Effect of microbial heat shock proteins on airway inflammation and hyperresponsiveness. J Immunol. 2002;169:5300–5307. doi: 10.4049/jimmunol.169.9.5300. [DOI] [PubMed] [Google Scholar]
- Rico AI, Angel SO, Alonso C, Requena JM. Immunostimulatory properties of the Leishmania infantum heat shock proteins HSP70 and HSP83. Mol Immunol. 1999;36:1131–1139. doi: 10.1016/S0161-5890(99)00136-4. [DOI] [PubMed] [Google Scholar]
- Rico AI, Girones N, Fresno M, Alonso C, Requena JM. The heat shock proteins, Hsp70 and Hsp83, of Leishmania infantum are mitogens for mouse B cells. Cell Stress Chaperones. 2002;7:339–346. doi: 10.1379/1466-1268(2002)007<0339:THSPHA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanello NC, Barber BL, Reiss TF, Friedman BS, Juniper EF, Zhang J. Measurement characteristics of two asthma symptom diary scales for use in clinical trials. Eur Respir J. 1997;10:646–651. [PubMed] [Google Scholar]
- Silva E, Bourin S, Sabounchi-Schutt F, Laurin Y, Barker E, Newman L, Eriksson H, Eklund A, Grunewald J. A quantitative proteomic analysis of soluble bronchoalveolar fluid proteins from patients with sarcoidosis and chronic beryllium disease. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:24–32. [PubMed] [Google Scholar]
- Simpson JL, McElduff P, Gibson PG. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration. 2010;79:147–151. doi: 10.1159/000245899. [DOI] [PubMed] [Google Scholar]
- Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- Stice JP, Knowlton AA. Estrogen, NFkappaB, and the heat shock response. Mol Med. 2008;14:517–527. doi: 10.2119/2008-00026.Stice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamási L, Bohacs A, Tamási V, Stenczer B, Prohaszka Z, Rigo JJ, Losonczy G, Molvarec A. Increased circulating heat shock protein 70 levels in pregnant asthmatics. Cell Stress Chaperones. 2010;15:295–300. doi: 10.1007/s12192-009-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Luo W. Heat shock proteins’ mRNA expression in asthma. Respirology. 2000;5:227–230. doi: 10.1046/j.1440-1843.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- Vignola AM, Chanez P, Polla BS, Vic P, Godard P, Bousquet J. Increased expression of heat shock protein 70 on airway cells in asthma and chronic bronchitis. Am J Respir Cell Mol Biol. 1995;13:683–691. doi: 10.1165/ajrcmb.13.6.7576706. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Toledo AH, Rivera-Chavez FA, Lopez-Neblina F, Toledo-Pereyra LH. Inflammatory mediators of liver ischemia-reperfusion injury. Exp Clin Transplant. 2009;7:78–93. [PubMed] [Google Scholar]
- Wheeler DS, Chase MA, Senft AP, Poynter SE, Wong HR, Page K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir Res. 2009;10:31. doi: 10.1186/1465-9921-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy. 2009;39:12–19. doi: 10.1111/j.1365-2222.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- Yang M, Wu T, Cheng L, Wang F, Wei Q, Tanguay RM. Plasma antibodies against heat shock protein 70 correlate with the incidence and severity of asthma in a Chinese population. Respir Res. 2005;6:18. doi: 10.1186/1465-9921-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]