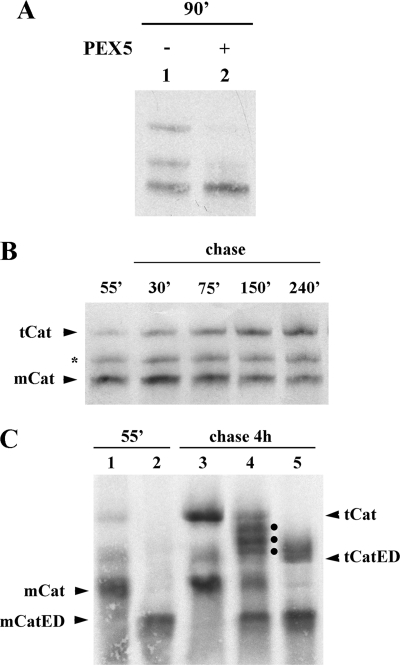
FIGURE 1.
35S-Labeled catalase tetramerizes in vitro. A, human catalase was synthesized in vitro in a rabbit reticulocyte lysate for 90 min at 30 °C in the absence or presence of 1 μm human PEX5, as indicated, and analyzed by native-PAGE/autoradiography. B, 35S-labeled catalase was synthesized for 55 min. After adding cycloheximide, an aliquot was removed and frozen in liquid N2 (lane 55′). The remainder of the reaction was then incubated at 30 °C, and aliquots were removed and frozen at the indicated time points. The samples were subjected to native-PAGE/autoradiography. The protein bands labeled with mCat and tCat correspond to the monomeric and tetrameric forms of catalase; the band labeled with an asterisk probably represents dimeric catalase (see text for details). C, catalase and a mutant version of it possessing two acidic amino acid residues at the C terminus (CatED) were synthesized in vitro for 55 min and supplemented with cycloheximide (lanes 1 and 2, respectively). Aliquots of each reaction were then combined and incubated for 4 h at 30 °C (lane 4) or incubated individually under the same conditions (lanes 3 and 5 for catalase and CatED, respectively), and subjected to native PAGE/autoradiography. Note that this gel was run for 2.5 h to improve separation of tetramers. Longer electrophoretic runs also result in more diffuse bands. The dots in lane 4 indicate the three expected heterotetramers. mCatED and tCatED indicate the monomeric and tetrameric forms of CatED, respectively.