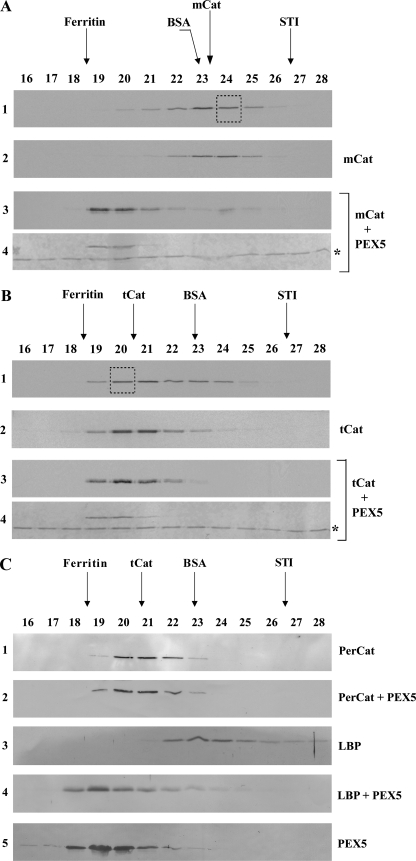
FIGURE 2.
PEX5 binds monomeric catalase. A, 35S-labeled catalase was synthesized in vitro for 55 min and subjected to SEC. Radiolabeled mCat eluting in fraction 24 of this chromatography (panel 1, boxed lane) was then subjected to a second SEC either alone (panel 2) or after receiving 1 μm recombinant PEX5 (panels 3 and 4). Fractions were collected and subjected to SDS-PAGE/Western blotting. Autoradiographs (panels 1–3) and the Ponceau S-stained membrane showing PEX5 (panel 4) are presented. No recombinant PEX5 or 35S-labeled catalase were detected in the void volume of this column (fractions 14 and 15; not shown). The asterisk marks bovine serum albumin added to chromatography fractions before precipitation to control protein recoveries. B, 35S-labeled catalase, synthesized in vitro for 55 min and incubated for 4 h at 30 °C in the presence of cycloheximide, was subjected to SEC. Radiolabeled tCat eluting in fraction 20 (panel 1, boxed lane) was then subjected to a second SEC either alone (panel 2) or after receiving 1 μm recombinant PEX5 (panels 3 and 4). Fractions were processed as described above. Autoradiographs (panels 1–3) and the Ponceau S-stained membrane (panel 4) are presented. C, soluble proteins from mouse liver peroxisomes were incubated either with recombinant PEX5 or buffer alone and subjected to SEC. Fractions were subjected to SDS-PAGE/Western blotting using antibodies directed to catalase (PerCat) or L-bifunctional protein. Immunoblots (panels 1–4) and a Ponceau S-stained membrane showing PEX5 (panel 5) are presented. Note that PEX5, a monomeric 70-kDa protein in solution, displays an abnormal behavior upon SEC because a major fraction of its polypeptide chain is natively unfolded (52).