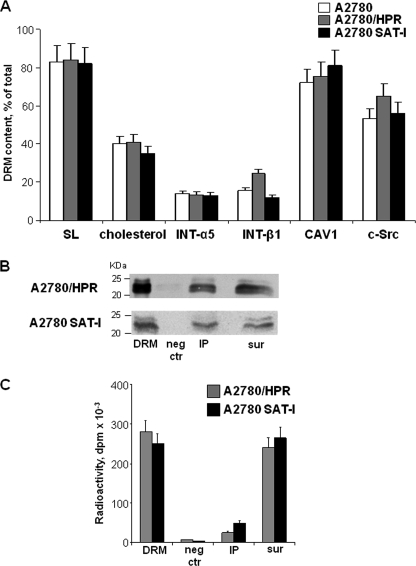
FIGURE 6.
Immunoprecipitation of caveolin-1 from a Triton X-100-resistant fraction from A2780, A2780/HPR, and SAT-1-transfected A2780 cells. Panel A, lipid and protein composition of the detergent-insoluble membrane fractions. After metabolic labeling of cell lipids with [1-3H]sphingosine, DRM fractions were prepared from A2780 (white bars), A2780/HPR (gray bars), and SAT-1-transfected A2780 cells (black bars) by sucrose gradient centrifugation after lysis in the presence of 1% Triton X-100 as described under “Experimental Procedures.” Lipids from the DRM fraction and from the other gradient fractions were extracted in chloroform/methanol and separated by HPTLC. Radioactive sphingolipids were detected and quantitatively analyzed by digital autoradiography, and cholesterol was detected colorimetrically and quantitatively analyzed by densitometry. Data are expressed as the percentage amount of total sphingolipids (SL) and cholesterol associated with the DRM fractions with respect to the total amount in the gradient. DRM and other gradient fractions have been also analyzed by SDS-PAGE followed by Western blotting detection using specific antibodies against β1 integrin, α5 integrin, caveolin-1, and c-Src. The relative quantities of each protein in the fraction were calculated by densitometry on the basis of the intensity of the blotting signal. Data are expressed as percentage of total signal associated with each antigen in the cell lysates. Data are the means ± S.D. of three independent experiments. Panel B, immunoprecipitation of caveolin-1 from DRM obtained from of A2780/HPR and SAT-I-transfected A2780 cells. Immunoprecipitation experiments using anti-caveolin-1 monoclonal antibody were performed starting from the same DRM amount under domain preserving conditions in which the interactions between lipid and protein within the plasma membrane have been maintained. Caveolin-1 was detected in each sample by Western blotting. 1st lane, DRM (1/30 of total volume). 2nd lane, negative control (neg ctr) (1/10 of the total volume). 3rd lane, anti-caveolin-1 immunoprecipitate (IP) (1/10 of the total volume). 4th lane, supernatants after immunoprecipitation (sur) (1/40 of total volume). Panel C, after metabolic labeling of cell sphingolipids with [1-3H]sphingosine, radioactivity-associated sphingolipids in immunoprecipitation samples obtained from of A2780/HPR and SAT-I-transfected A2780 cells (1st lane, whole DRM; 2nd lane, negative control; 3rd lane, anti-caveolin-1 immunoprecipitate; 4th lane, supernatants after immunoprecipitation) have been determined as described above. Data are expressed sphingolipid-associated radioactivity in each sample and are the means ± S.D. of three independent experiments.