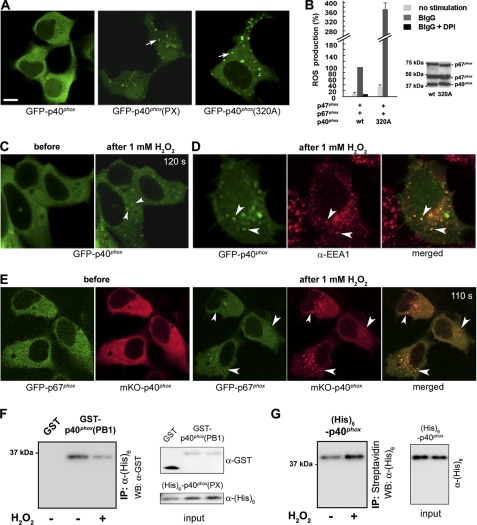
FIGURE 1.
H2O2 induces EE targeting of p40phox. A, transfected GFP-p40phox is localized in the cytoplasm (left); in contrast, GFP-p40phox(PX) (center; arrow) and p40phox(F320A) (right; arrow) are localized at dotlike structures, in resting WT HEK293 cells. Bar, 10 μm. B, p40phox(F320A) co-expressed with p47phox + p67phox supports moderate constitutive (32.0 ± 6.2%) and high BIgG-stimulated ROS production in HEK293Nox2/FcγRIIa cells when compared with cells expressing WT p40phox + p47phox + p67phox. Western blotting detects comparable levels of cytoplasmic Phox proteins in both transfection experiments. C, in response to exogenous 1 mm H2O2 (120 s after stimulation), GFP-p40phox expressed in WT HEK293 cells translocates to dotlike structures (arrowheads). D, the dotlike structures are co-stained by an EE marker, EEA1 (arrowheads). E, cytoplasmic GFP-p67phox co-expressed with mKO-p40phox in WT HEK293 cells translocates to dotlike structures (arrowheads) after stimulation (110 s) with 1 mm H2O2. Similar effects of H2O2 are observed in RAW 264.7 cells (supplemental Fig. 3). F, effect of H2O2 on the PX-PB1 domain interaction in vitro. The interaction between purified His6-p40phox(PX) and GST-p40phox(PB1) proteins detected by pull-down assays is weakened by the addition of 0.01 mm H2O2. Similar results are obtained in three independent experiments. G, effect of H2O2 on binding of p40phox to PI(3)P in vitro. The interaction between purified full-length His6-p40phox protein and PI(3)P-containing liposomes detected by pull-down assays is strengthened by the addition of 0.01 mm H2O2. Similar results were obtained in three independent experiments. Error bars, S.E.