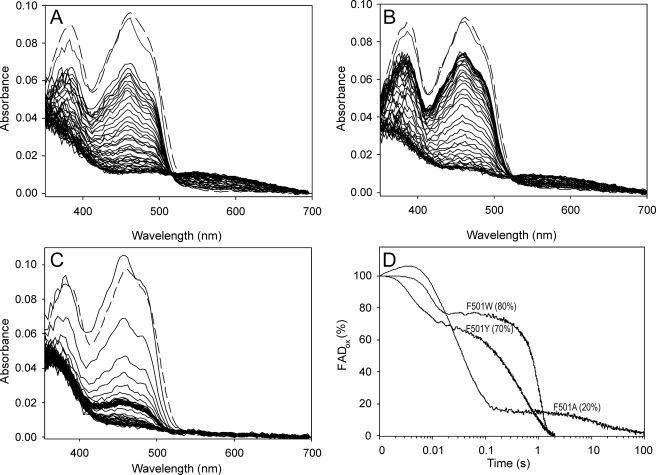
FIGURE 4.
Spectral changes during F501Y (A), F501W (B), and F501A (C) turnover with p-methoxybenzyl alcohol under an air atmosphere and time course of the above reactions followed at 462 nm (D). An aerobic solution of enzyme (∼10 μm) was reacted in the stopped-flow instrument with 0.6 mm p-methoxybenzyl alcohol (under an air atmosphere in 0.1 m phosphate (pH 6) at 12 °C). The oxidized spectrum before the reaction is indicated by dashed lines, and the first reaction spectrum was recorded 2 ms after mixing. Then, spectra are shown every 20 ms in the 2–162-ms range and then every 50 ms in the 0.162–2-s range (and every 5 s in the 2–125-s range in C). Native AAO (not shown) showed spectral changes similar to those of the F501Y variant (A). The time course of the reactions (D), shown as a percentage of the oxidized form on a logarithmic time scale, revealed different enzyme oxidation degrees (20–80%) under steady-state turnover (attained after a variable-length initial decrease and before O2 exhaustion in the reaction) according to the different O2 reactivities.