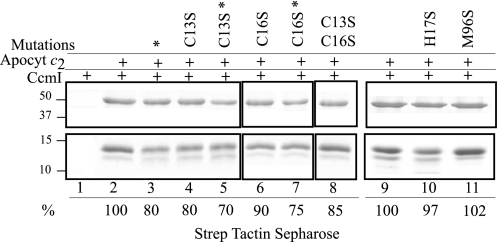
FIGURE 6.
Co-purification of CcmI with apocytochrome c2 and its derivatives lacking the heme binding site Cys residues Cys-13 and C-16 or the heme iron axial ligands His-17 and Met-96. Co-purification experiments were conducted as in Fig. 3, panel B, except that His10-CcmI (5 μg) was incubated with apocytochrome c2 (15 μg) or its appropriate derivatives. Lane 1 shows that CcmI does not exhibit unspecific binding to Strep Tactin resin. Lanes 2 and 9 show co-purification of CcmI with native apocytochrome c2, and the amount of CcmI present in the elution fraction was taken as 100% for image quantification using Image J program and used for comparison with the amounts seen in lanes 3–8, 10, and 11. Lanes 4, 6, and 8 show slightly lower amounts of CcmI present in the elution fractions when apocytochrome c2 (Apocyt c2)-C13S (80%), -C16S (90%), and -C13S/C16S (85%), respectively, were used. Lanes 3, 5, and 7 also show lower amounts of CcmI present in the elution fractions when wild type apocytochrome c2 (80%) and single Cys mutants (70% for C13S and 75% for C16S) treated with DTT and IAM, respectively, were used. Lanes 10 and 11 show similar amounts of CcmI present in the elution fractions when incubated with apocyt-H17S (97%) and -M96S (102%) mutants, respectively, as compared with native apocytochrome c2 (lane 9). The asterisks indicate that apocytochrome c2 Cys thiols were treated with DTT/IAM, and the molecular markers (in kDa) are indicated on the left.