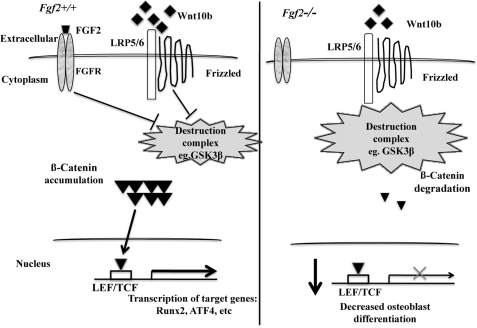
FIGURE 6.
Schematic model in which FGF2 stimulation of osteoblast differentiation is partially through modulation of the Wnt/β-catenin pathway. Loss of endogenous FGF2 results in reduced Wnt10b mRNA expression, reduced Lrp5/Lrp6 mRNA expression, reduced β-catenin mRNA and protein expression, and reduced inactive phosphorylated GSK3β protein expression, all of which lead to reduced Wnt/β-catenin signaling. Wnt/β-catenin signaling directly regulates osteoblast transcription factors, including Runx2 and probably ATF4. The reduced Wnt/β-catenin signaling and reduction of Dkk2 mRNA expression (important for terminal osteoblast differentiation) probably contribute to decreased osteoblast differentiation and bone formation in Fgf2−/− mice. However, exogenous FGF2 promoted β-catenin nuclear accumulation and induced inhibited kinase GSK3β, which could increase Wnt signaling in Fgf2−/− BMSCs and probably contributes to rescue the decreased osteoblast differentiation in Fgf2−/− BMSCs. LEF/TCF, lymphoid enhancer-binding factor 1/T cell-specific transcription factor.