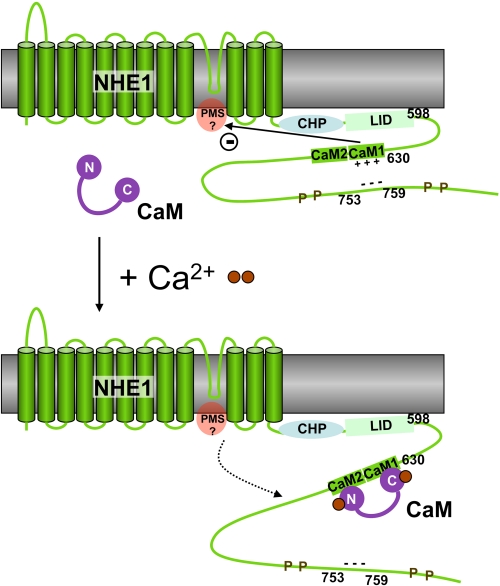
FIGURE 6.
Schematic model of the calcium-dependent regulation of NHE1 by CaM. The antiporter activity of NHE1 is down-regulated by the autoinhibitory domain within the CaM binding region (CaM1 and CaM2), probably by its interaction with the proton modifier site (PMS). The positive charges of CaM1 are neutralized by the binding of an acidic region in the downstream region 753–759, which is flanked by several phosphorylation sites (P). Upon the binding of the secondary messenger Ca2+ (brown spheres), CaM is able to bind to NHE1CaMBR, thus replacing the 753–759 region. This would counteract the autoinhibitory domain, resulting in an up-regulation of the NHE1 antiporter activity. CHP, calcineurin B homologous protein. LID, lipid-interacting domain.