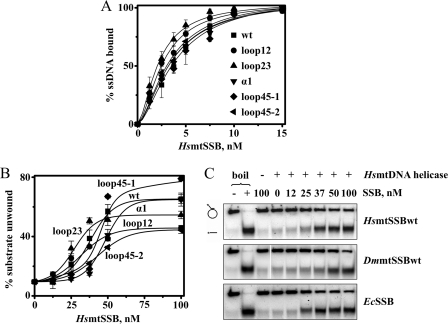
FIGURE 2.
ssDNA binding affinities and stimulation of HsmtDNA helicase by HsmtSSB variants. A, ssDNA-binding affinity was evaluated by GMSA, as described under “Experimental Procedures.” The fraction of unbound and bound oligomer was quantitated, and the data were plotted as the average percent of substrate utilized from three independent experiments. B and C, DNA unwinding assays were performed as described under “Experimental Procedures,” using 3.5 nm of HsmtDNA helicase (as hexamer). “−” and “+ boil” lanes represent the intact and denatured (heated to 100 °C for 2 min prior to loading) substrate, respectively. The data in B represent the average of unwound substrate as percent from three independent experiments.