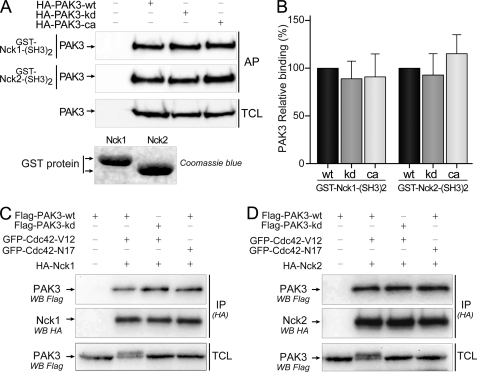
FIGURE 3.
PAK3 binds Nck2 independently of its kinase activity. A, in vitro interaction of different PAK3 kinase mutants with the second SH3 domains of Nck1 and Nck2. WT, kinase-dead (kd), and constitutively active (ca) forms of HA-PAK3 were expressed in COS-7 cells, and lysates were incubated with recombinant GST proteins containing the second SH3 domain of Nck1 (1st panel) or Nck2 (2nd panel). Affinity-purified (AP) PAK3 proteins and COS-7 expressed PAK3 proteins (TCL) were revealed by Western blotting (WB) with HA antibodies. Data shown are representative of a typical experiment from three independent experiments. Amount of GST proteins was controlled by Coomassie Blue staining. Note that the lower electrophoretic migration of the GST-Nck1-(SH3)2 protein is due to the presence of an HA tag. B, quantification of the amount of different PAK3 proteins precipitated with the second SH3 domain of Nck adaptors from the experiments illustrated in A, n = 3. Error bars indicate the S.E. C and D, Cdc42 activation of PAK3 does not suppress its interaction with Nck1 (C) or Nck2 (D). COS-7 cells were co-transfected with plasmids coding for FLAG-PAK3-WT or FLAG-PAK3-kd (kinase-dead), with either active Val-12 or inactive Asn-17 mutants of GFP-Cdc42 vectors, together with HA-tagged Nck1 (C) or Nck2 (D) plasmids. Nck1 or Nck2 proteins were HA-immunoprecipitated (IP (HA)) and visualized after HA immunoblotting (middle panels). PAK3 proteins in immune complexes (upper panels) and in TCLs were revealed by anti-FLAG immunoblotting. GTPase expression was verified using anti-GFP antibody (data not shown). Note that activation of PAK3 by Cdc42-Val-12 induced an electrophoretic shift of PAK3-WT protein as previously reported (25).