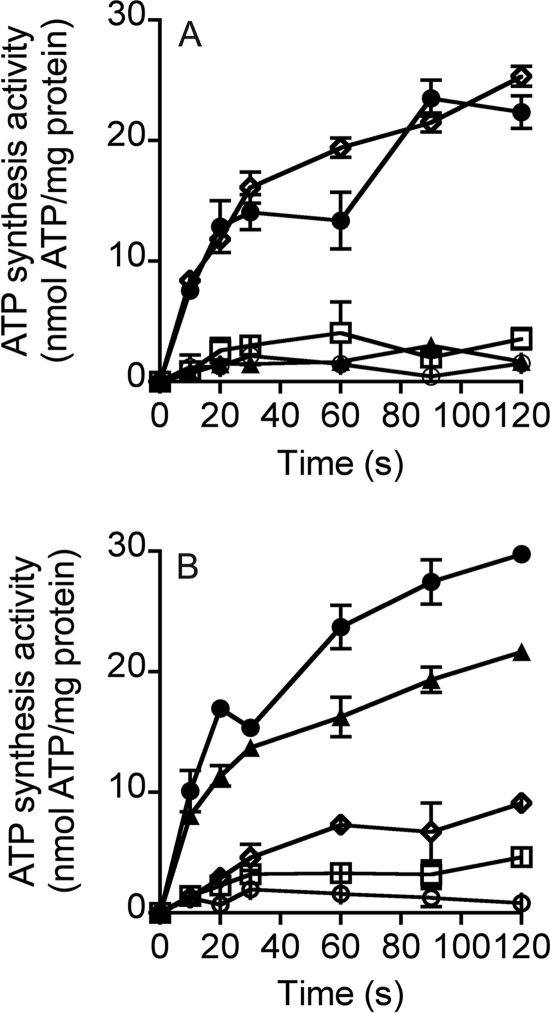
FIGURE 6.
ATP synthesis properties of the MbbrA1Ao-ATP synthase in inverted membrane vesicles from E. coli DK8 (Δatp). Time course ATP synthesis assays at pH 6.5 at 30 °C with 0.5 mg of inverted membrane vesicles. A, ATP synthesis was initiated by the addition of a valinomycin (2 μm)-induced potassium diffusion potential of >100 mV (solid circles), no valinomycin addition (open circles), CCCP (100 μm, solid triangles), TBT-Cl (150 μm, open squares), and monensin (5 μm, open diamonds). B, effect of ΔpNa+ on ATP synthesis by inverted membrane vesicles. To generate a ΔpNa+, sodium-loaded vesicles (125 mm NaCl) were diluted 40-fold into buffer A (50 mm MES (pH 6.50), 40 mm potassium acetate, 40 mm K2S2O5, 10 mm MgSO4, 160 mm KCl, 1.5 mm ADP, 5 mm KH2PO4) containing one of the following: 3.1 mm NaCl (inside NaCl concentration, 125 mm) to generate a ΔpNa+ of 95 mV (open circles); 3.1 mm NaCl and 2 μm valinomycin to generate a generate a ΔpNa+ of 95 mV and a K+/valinomycin diffusion potential of >100 mV (solid circles); 5 μm monensin, 3.1 mm NaCl, and 2 μm valinomycin to generate a generate a ΔpNa+ of 95 mV and a K+/valinomycin diffusion potential of >100 mV (open diamonds); 100 μm CCCP, 3.1 mm NaCl, and 2 μm valinomycin to generate a generate a ΔpNa+ of 95 mV and a K+/valinomycin diffusion potential of >100 mV (solid triangles); 150 μm TBT-Cl, 3.1 mm NaCl, and 2 μm valinomycin to generate a generate a ΔpNa+ of 95 mV and a K+/valinomycin diffusion potential of >100 mV (open squares). Each point is the result of three technical replicates, and the standard error associated with this measurement is shown.