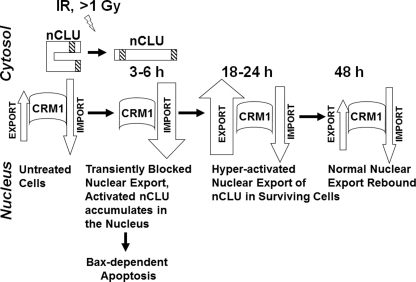
FIGURE 7.
Model for nuclear import/export of the nCLU cell death protein in control and IR-exposed cells. We propose that in untreated cells, a majority of nCLU is in an inactive conformation as pnCLU, a form most likely folded onto itself to hide its functional NLS. Under normal growth conditions, a low level of expressed and active mature ∼55-kDa nCLU binds CRM1. Most likely an intermediate carrier protein is involved, and this complex is actively exported out of the nucleus. This mechanism of export maintains critical balanced levels of nCLU and prevents cell death. Exposure to IR (≥1 Gy) causes activation of nCLU via a currently unknown mechanism. The net result is the inability of CRM1 to bind activated nCLU after IR, resulting in accumulation of nCLU in the nuclei of cells, which enhances cell death and inhibits double strand break DNA repair. By this mechanism, the nuclear import/export balance of activated nCLU is shifted toward import. nCLU activation, in turn, disrupts Ku70-Bax complexes, resulting in Bax release. This mechanism appears to explain the resistance to nCLU-mediated cell death in bax-deficient cells. Activated nCLU also binds BH3 domain proteins, sequestering pro-survival proteins such as Bcl-Xl.