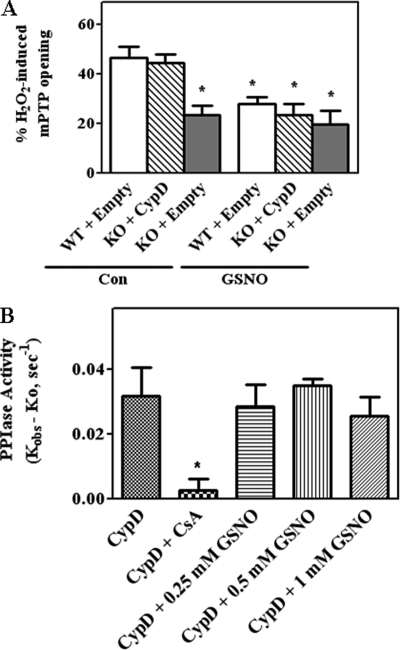
FIGURE 2.
H2O2-induced mPTP opening is blocked by GSNO. In A, CypD WT MEFs were transfected with pCMV-XL6 (control (Con) plasmids). CypD−/− MEFs (KO) were transfected either with pCMV-XL6 (control plasmids) or WT CypD for 48 h as labeled in each panel. After transfections, mPTP opening was assessed using H2O2 in the presence and absence of a NO donor, GSNO, using the calcein-cobalt quenching technique. *, p < 0.05 versus WT+empty or KO+CypD (n = 5). In B, PPIase activity was measured using recombinant CypD in the presence of 0, 0.25, 0.5, and 1 mm GSNO. The reaction was initiated with PPIase substrate and chymotrypsin as described under “Experimental Procedures.” The kobs for PPIase activity was calculated using non-linear regression analysis as described under “Experimental Procedures.” The k0 is the PPIase activity in the absence of CypD (the non-enzymatic rate) was also calculated using non-linear regression and was subtracted from the kobs. The results represent average measurements from three independent assays.