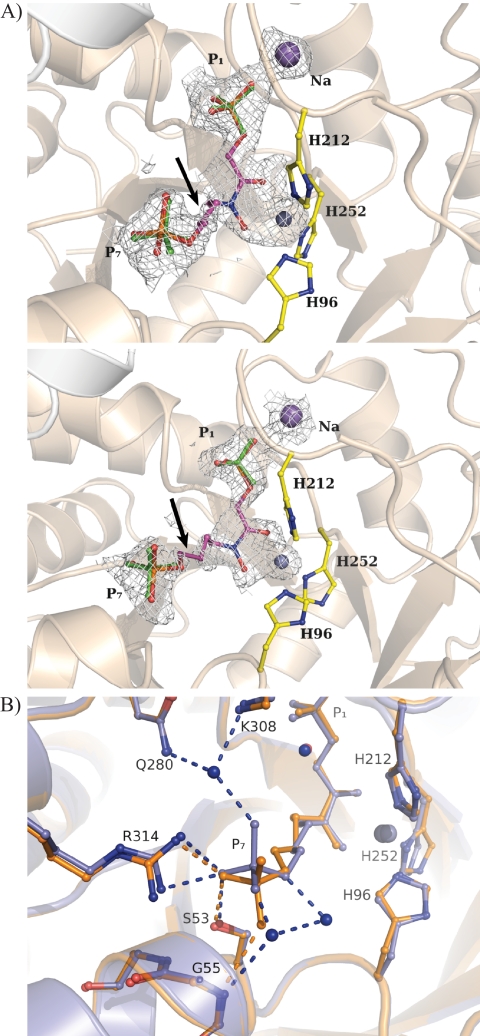
FIGURE 5.
Binding by substrate analog TD3 in FBA-tb subunits. A, difference electron density (Fo − Fc) simulated annealed omit map showing fit to the electron density map by inhibitor TD3 bound in the active site of FBA-tb. The subunit corresponding to high occupancy binding (0.6) by TD3 is shown in the top panel, whereas that of low occupancy binding (0.3) is shown in the middle panel. P1 and P7 phosphates of TD3 are identified. Residual electron density at the P1 and P7 phosphates binding loci was refined as sulfate ions that are shown in green. Side chains of the histidine residues chelating the catalytic zinc ion are also depicted. The figure was drawn and the superimposition was prepared using the program PyMOL. The catalytic zinc ion is shown as a gray sphere in all figures. TD3 binding further activates sodium ion binding with the P1 oxyanion. The arrows point to the C5-C6 bond in TD3 that corresponds to the weakest region of electron density in the difference map for TD3 in both subunits. The electron density of the difference omit, which is shown outlining the active binding event, was set to the 2σ level instead of 4σ to reflect the partial occupancy by TD3 and metal ion in the active site. B, superimposition of FBA-tb subunits binding the TD3 inhibitor. The TD3 binding locus is identical in the two subunits. The inhibitor bound in the high occupancy subunit is shown in blue, and it is shown in orange for the low occupancy subunit. In the subunit corresponding to high TD3 occupancy (0.6), the P7 oxyanion participates in three well defined hydrogen bonding interactions with water molecules that are not present in the subunit exhibiting lower TD3 occupancy (0.3). The presence of solvating water molecules in the high occupancy subunit and their absence in the other subunits were corroborated by electron density omit maps (data not shown). The same binding geometry corresponding to the high occupancy TD3 site was also observed for zinc ion chelating FBP in FBA-tb (19). The extensive superimposition of FBP and TD3 molecules in the FBA-tb active site shown in supplemental Fig. S4 reinforces the role of TD3 as a competitive inhibitor. Binding by TD3, however, does not induce any additional conformational changes with respect to active site binding by FBP.