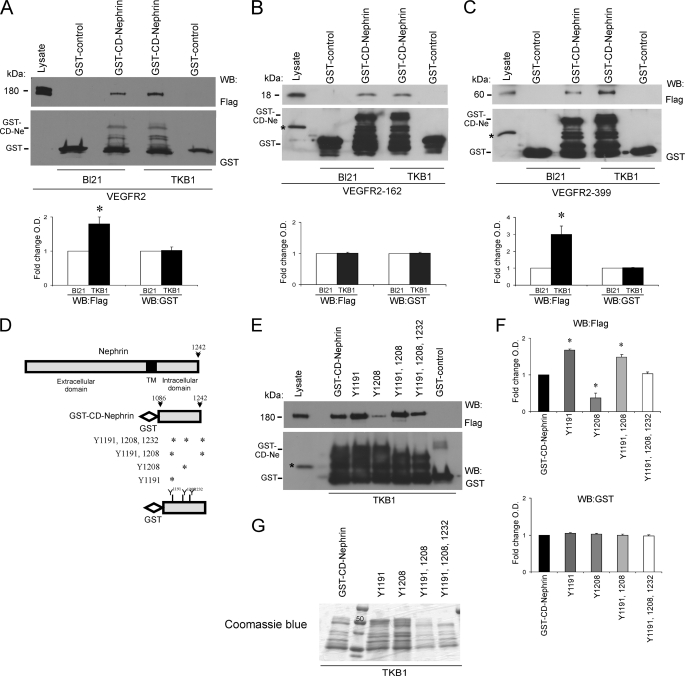
FIGURE 5.
VEGFR2-nephrin interaction is modulated by tyrosine phosphorylation of nephrin cytoplasmic domain. A, in vitro GST-binding assays with purified GST-CD-nephrin incubated with purified full-length FLAG-VEGFR2. Tyrosine phosphorylation of CD-nephrin (Ne; TKB1) increases the direct interaction with VEGFR2, as compared with non-phosphorylated CD-nephrin (BL21). B and C, GST-binding assay shows that nephrin tyrosine phosphorylation (TKB1) enhances the interaction with VEGFR2-399, whereas it does not alter the interaction with VEGFR2-162. GST alone was used as negative control. Bar graphs show quantification of densitometric analysis of four independent experiments; data were normalized for BL21 and expressed as mean ± S.E. *, p < 0.05. D, schemes depict full-length nephrin, the predicted transmembrane domain (TM), GST-CD-nephrin, and mutation analysis of CD-nephrin tyrosine residues; asterisks indicate the residue(s) substituted by phenylalanine in each of the four constructs. E, GST binding assays showed that the association between purified VEGFR2 and phosphorylated CD-nephrin decreased when Tyr-1208 was mutated, and increased when Tyr-1191 was mutated. F, quantitation of densitometric analysis of three independent experiments, normalized for GST-CD-nephrin and expressed as mean ± S.E. An asterisk indicates p < 0.05. G, representative Coomassie Blue gel of GST-CD-nephrin and mutants in TKB1 bacteria.