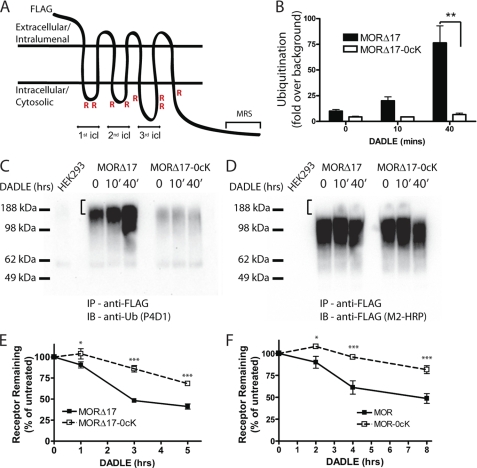
FIGURE 2.
MOR down-regulation measured radioligand binding requires receptor ubiquitination. A, shown is a diagram of the F-MOR-0cK construct indicating cytoplasmic domains containing the Lys → Arg mutation (R) as well as the MRS that is devoid of lysine residues. icl, cytoplasmic loop. B, shown is a densitometric analysis of ubiquitin incorporation into the F-MORΔ17 and the lysyl-mutant F-MORΔ17–0cK, assessed after immunopurification of receptors in the presence of SDS. Data shown represent the mean and S.E. of densitometry from anti-ubiquitin immunoblot analysis carried out in n = 3 independent experiments. Scanning densitometry was carried out in the linear range and is expressed as -fold over background (defined by nonspecific signal detected in parental HEK293 cells not expressing the indicated FLAG-tagged receptor). C, a representative anti-ubiquitin immunoblot (IB) from the analysis summarized in panel B; IP, immunoprecipitate. D, shown is the same blot as in panel C, except it was stripped and reprobed with anti-FLAG to verify comparable loading and transfer of immunopurified receptors. E and F, shown is the effect of the indicated lysyl mutations on DADLE-induced down-regulation. HEK293 cells stably expressing F-MORΔ17 or F-MORΔ17–0cK (panel E) or F-MOR or F-MOR-0cK (panel F) were exposed to 10 μm DADLE for the indicated time period before estimating Bmax by radioligand binding to [3H]DPN. Points represent specific binding at each time point, expressed as a percentage of the specific binding in cells not exposed to agonist. In each experiment triplicate tubes were analyzed; points represent averages and error bars S.E. across experiments (n = 4; *, p < 0.05; ***, p < 0.001, two way ANOVA).