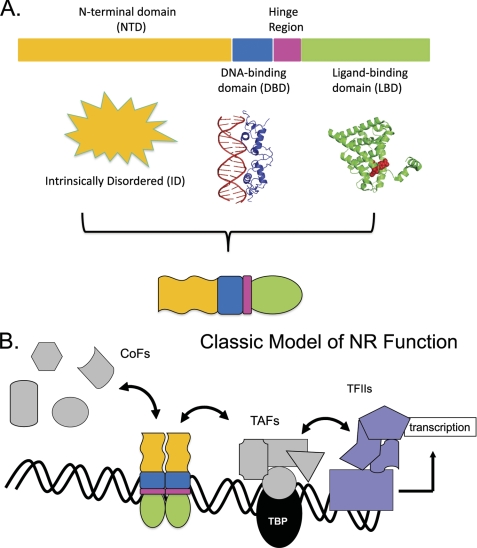
FIGURE 1.
Classic model of SHR function. A, structural architecture: SHRs are composed of three domains with segregated functional roles. The globular C-terminal LBD (green) binds steroidal or other small ligands. This alters the position of the LBD 12th helix to configure the AF2 surface so as to bind “ligand-dependent” CoFs containing LXXLL motifs. The globular DBD (blue) recognizes specific DNA sequences. The hinge region (pink) connects the DBD and LBD. The NTD (yellow; structure undetermined) is of varying length and sequence in the SHR family of TFs. In some NRs, it is absent altogether. When present, the NTD may bind CoFs without requirement for an LBD ligand (“ligand-independent”). The AF1 regions of the NTD vary in potency for regulating transcription. B, functional scheme: binding of steroid localizes the SHR to the nucleus. SHRs homodimerize and other NRs heterodimerize and associate with their RE DNA sequences. The NR-DNA complex attracts CoFs and other proteins of the transcriptional and chromatin-modifying apparatus. TBP, TATA box-binding protein; TAFs, TBP-binding factors; RNA Pol II, RNA polymerase II.