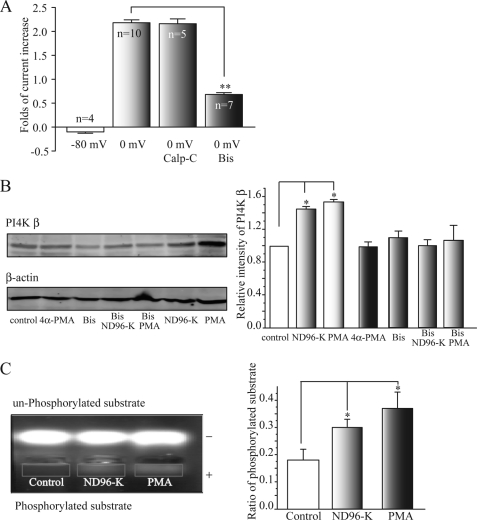
FIGURE 2.
PKC is involved in the depolarization-induced increased activity of PI4 kinase β. A, PKC blocker Bis significantly inhibited the depolarization (0 mV)-induced potentiation of KCNQ2/Q3 currents. Bis (1 μm) was applied for 2 h before PMA (50 nm) application. Calp-C did not inhibit the potentiation. **, p < 0.01 compared with the 0 mV group. B, bisindolylmaleimide blocked the depolarization- (ND-96K) and PMA-induced enhancement of PI4K β expression. Western blot band intensity was first normalized to the corresponding β-actin intensity and then was normalized again to the control. *, p < 0.05 compared with the control. C, depolarization (ND96-K) or PMA increases PKC activity. The oocytes were exposed to ND96-K for 15 min or PMA (50 nm) for 20 min. PKC activity, as demonstrated by the density of the downward-shifted PKC substrate peptide, was increased by incubation with either ND96-K or PMA. The phosphorylated peptide that served as a PKC substrate migrated toward the cathode (+), while the nonphosphorylated peptide migrated toward the anode (−). The right panel shows summary data for the ratio of phosphorylated substrate (band intensity of phosphorylated substrate (rectangle)/unphosphorylated) from seven independent experiments. *, p < 0.05 compared with the control.