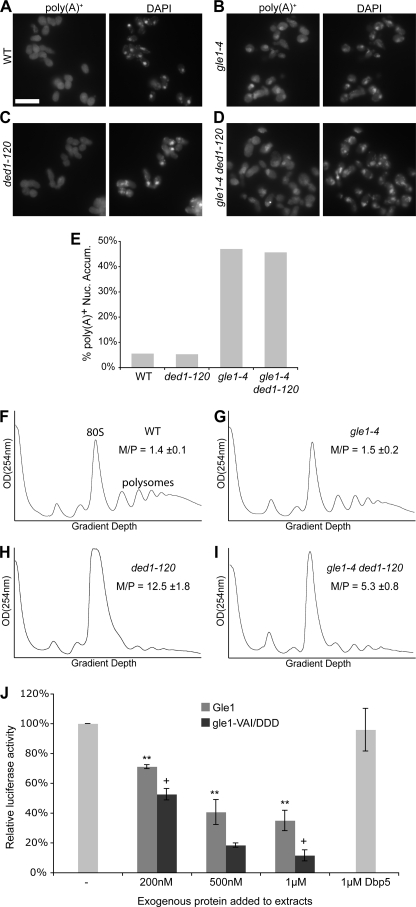
FIGURE 4.
The Gle1:Ded1 interaction is linked to translation initiation. A–D, poly(A)+ RNA was localized by in situ hybridization with oligo-dT probes in wild-type (WT), gle1–4, ded1–120, and double mutant strains after shifting to 30 °C for two hours. Representative images are shown. Size bar in A corresponds to 10 μm. E, quantification of in situ results as the percentage of cells displaying nuclear accumulation of poly(A)+ signal. Cells were scored as positive if the nuclear poly(A)+ signal substantially exceeded cytoplasmic signal. Counts represent n >100 cells for each sample from at least five fields. F–I, cultures of indicated strains were shifted to 20 °C for 1 h, and polysome profiles were generated by subjecting cell lysates to 7–47% sucrose density centrifugation and OD254 nm analysis of the gradient. Monosome/polysome (M/P) ratio was determined by comparing the area of the monosome peak to the sum of the areas of the first four polysome peaks. Error represents S.E. from n = 3. The monosome (80 S) and polysome peaks are labeled in F for reference. J, for in vitro translation assays, competent extracts from wild-type cells were incubated with in vitro-transcribed luciferase mRNA, and luciferase reactions were performed. Purified Gle1, gle1-VAI/DDD, and Dbp5 were added to the reactions as indicated.