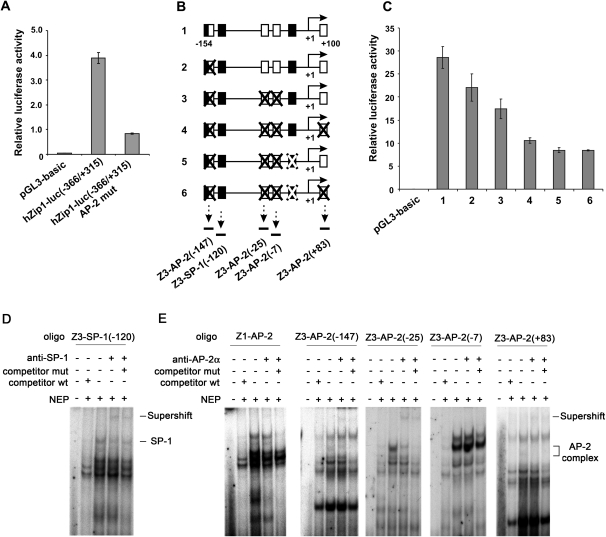
Fig. 2.
The normal prostate epithelial cell line RWPE-1 was transfected with the pGL3 luciferase reporter vectors. Twenty-four hours after transfection, luciferase activity was measured. Transcriptional activity of hZip1 and hZip3 promoter regions is represented by the histograms. The data are presented as the mean (±SD) of the three independent experiments performed in triplicates. (A) Transcriptional activity of the wild-type hZip1 (−331/+367) promoter region and its analog with a mutated AP-2-binding site. (B) Schematic illustration of native hZip3 (−193/+68) promoter region fragments and its mutated analogs cloned into the pGL3 luciferase reporter vector. Black squares represent SP1 binding sites, and white squares represent AP-2 binding sites. Mutated binding sites are crossed. Arrows with dotted lines under binding sites represent localization of the double-stranded DNA oligos used in Gel-Supershift assays displayed on panels D and E. (C) Transcriptional activity of hZip3 (−193/+68) promoter constructs indicated on panel B. (D and E) Gel-Supershift analysis of the ability of nuclear extract proteins (NEP) to bind double-stranded DNA oligos targeting the AP-2-binding site of the hZip1 promoter as well as the appropriate binding sites within the hZip3 promoter region indicated on panel B. NEP isolated from RWPE-1 cells were incubated with specific 32P-labelled double-stranded DNA oligos in the presence of 100-fold molar excess of their unlabeled wild-type (wt), mutant (mut) competitors or alone. Anti-AP-2alpha or anti-SP-1 antibodies were added to the NEP to demonstrate their binding specificity.