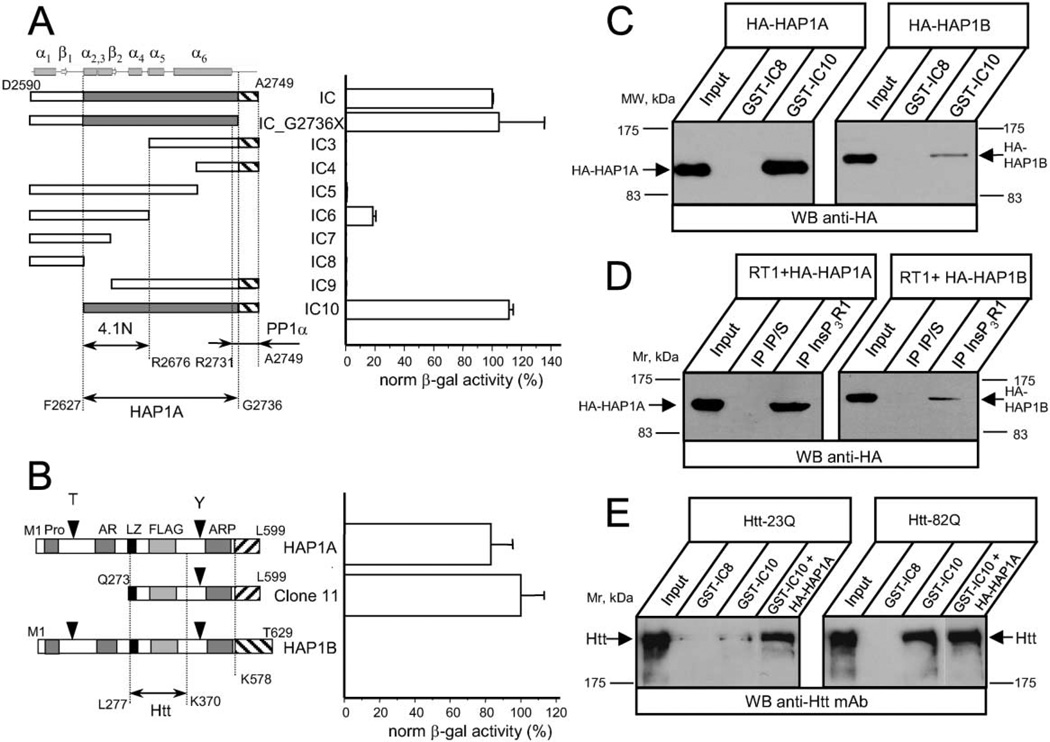
Figure 1. The InsP3R1 Binds HAP1A and Htt in the Yeast Two-Hybrid System and In Vitro.
(A) The strength of interaction between each InsP3R1 IC bait and clone 11 (Q273–L599 of rat HAP1A) was determined by a liquid Y2H assay. The β-galactosiadase activity is plotted as a percentage relative to the IC/clone 11 interaction (mean ± SE, n = 3). On the top, predicted secondary structure elements within the IC sequence are shown. The predicted minimal fragment of the rat InsP3R1 carboxy terminus (F2627–G2736) required for association with HAP1A is shaded. The minimal 4.1N binding domain (F2627–R2676) (Maximov et al., 2003) and minimal PP1α binding region (R2731–A2749) (Tang et al., 2003) are also shown.
(B) The strength of interaction between full-length rat HAP1A, rat HAP1B, and clone 11 with IC10 InsP3R1 bait was determined by liquid Y2H assay. Data are presented in percentages relative to the observed interaction between clone 11 and IC10 (mean ± SE, n = 3). The carboxy-terminal regions unique to HAP1A and HAP1B isoforms are depicted by striped boxes. Also shown are the Htt binding region of HAP1 (L277–K370) (Li et al., 1995, 1998b) and motifs identified in HAP1 by computer analysis.
(C) GST-IC8/IC10 pull-down experiments of HA-HAP1A or HA-HAP1B from COS7 cells extracts.
(D) Lysates from RT1-infected Sf9 cells and COS7 cells overexpressing either HA-HAP1A or HA-HAP1B were mixed for 2 hr and analyzed by immunoprecipitation with anti-InsP3R1 polyclonal antibodies or corresponding preimmune sera (IP/S).
(E) GST-IC8/IC10 pull-down experiments of Htt-23Q or Htt-82Q from HEK293 cells extracts. COS7 lysate containing overexpressed HA-HAP1A was added to GST-IC10 pull-down reactions as indicated. The input lanes on (C)–(E) contain 1/50 of the COS7 and HEK293 cell lysates used in GST pull-down or immunoprecipitation experiments. The precipitated fractions were analyzed by Western blotting with anti-HA monoclonal antibodies (C and D), or anti-Htt monoclonal antibodies (E).