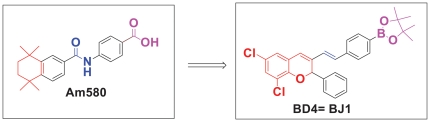
Figure 1. Synthesis of RARα agonist BD4.
We designed and synthesized a novel RARα agonist, BD4, by making a bridge of a functional group (trans double bond) between hydrophobic chromene rings and benzoic acid or acid isosteres. The functional group was designed not only to increase the efficacy but also to reduce the toxicity of compounds. These modifications also increase the specificity for retinoic acid receptor isoforms. Characteristic features of BD4 are as follows: a) Our compound BD4 is oxaretinoid; b) Incorporation of oxygen heteroatoms to replace one of the gem-dimethyl groups in the tetrahydronaphthalene ring of Am580 decrease the toxicity by retarding metabolic oxidation; c) To protect against protease-mediated hydrolysis of amides bonds found in Am580, we introduced trans-double bond as amide isosteres in BD4, which is more resistant to proteolysis; d) Substitution of boronic acid and ester in BD4 in place of acids generates a more biologically active molecular frameworks, which allows BD4 to interact with a target protein through both hydrogen bonds and covalent bonds. This interaction is predicted to produce more potent biological activity.