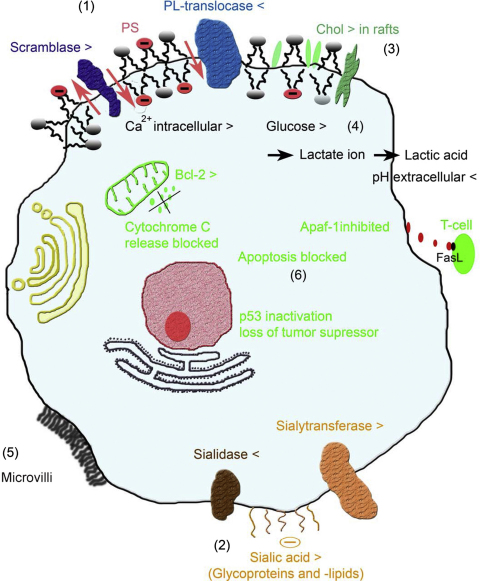
Fig. 2.
Sketch of specific characteristics of cancer cells concerning membrane and genetic changes. Selectivity of cationic anticancer peptides can be driven by direct interaction with anionic target molecules exposed on the cell surface such as PS. Exposure of PS (1) is related to several cell processes, which involves activation of a putative scramblase and inactivation of a putative ATP-dependent phospholipid (PL) – translocase. Increased levels of sialic acid of glycolipids and proteins on cancer surfaces (2), normally providing a protective shield, can also be a target for the positively charged peptides. Furthermore, changes in membrane fluidity (3) and pH (4) influence susceptibility and activity of peptides, which may also be affected by the increased surface area of cancer cells owing to the higher number of microvilli (5) present. Finally, peptides, which translocate into the cytosolic compartment, may interact with anionic lipids of mitochondria, triggering apoptosis, a process normally blocked by several changes in tumor cells such as e.g. inactivation of the tumor suppressor p53 (6). For details see main text.