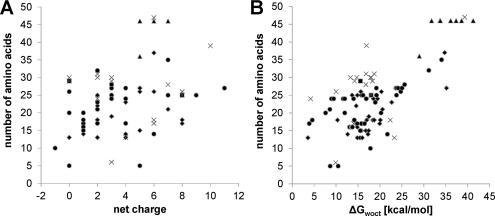
Fig. 6.
Pair correlations between peptide length and net charge (A) and hydrophobicity (B), respectively, for antitumor peptides listed in the Antimicrobial Peptide Database, APD2 (Wang et al., 2009b) excluding the three peptides >70 amino acids for which no ΔGwoct can be calculated. The secondary structure of the individual peptides is indicated in the panels by the following symbols: ♦, α-helix; ■, β-sheet; ▴, α-helix and β-sheet; ●, unknown; ×, others.