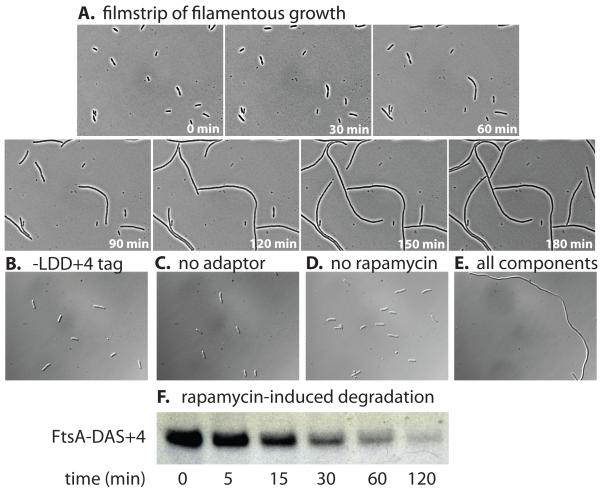
Figure 5. Rapamycin-dependent degradation of an essential cell division protein.
(A) E. coli W3110 sspB- bearing plasmid pJD427 was modified at the ftsA loci by addition of a DAS+4 tag fused to a kanamycin marker (JD784). This strain was grown in medium lacking rapamycin before cells were mounted on a microscope slide bearing LB/agar/rapamycin (10 μM) and imaged every 30 min. (B) E. coli W3110 sspB−/pJD427 with a non-degradable LDD+4 tag fused to FtsA exhibited normal cell morphology when grown in the presence of 10 μM rapamycin. Strain JD784 lacking the adaptor system (C) or grown in medium lacking rapamycin (D) also exhibited normal cell morphology. (E) Addition of both pJD427 and 10 μM rapamycin to strain JD784 resulted in a strong filamentation phenotype. (F) Western blotting shows intracellular degradation of FtsA-DAS+4 after addition of rapamycin (10 μM) in E. coli W3110 sspB−/pJD427. Protein synthesis was not blocked in this experiment. The sample volume in each lane was adjusted to contain the same amount of total cellular protein.