Abstract
Background:
Small-cell carcinoma (SCC) and large-cell neuroendocrine carcinoma (LCNEC) are uncommon in serous body cavity effusions. The purpose of this study is to examine the cytomorphological spectrum of SCC and LCNEC in body cavity serous fluids.
Materials and Methods:
We have 68 cases from 53 patients who had metastatic SCC or LCNEC diagnoses. All cytology slides and the available clinical data, histological follow-up, and ancillary studies were reviewed.
Results:
A total of 68 cases (60 pleural, 5 peritoneal, and 3 pericardial effusions) from 53 patients with an average age of 73 years (age range 43-92 years) were reported as diagnostic or suspicious of SCC (52 cases) or LCNEC (16 cases). The primary site was lung in 56 cases, pancreas in 6 cases, and 2 cases each from cervix, colon, and the head and neck region. Of the 68 cases, 48 cases had no history of malignancy of the same type. Ancillary studies were used in 46 cases (68%) including flow cytometric studies in 5 cases. There were three predominant cytomorphological patterns observed including small-cell clusters with prominent nuclear molding (33 cases, 49%), large-cell clusters mimicking non-small-cell carcinoma (18 cases, 26%), and single-cell pattern mimicking lymphoma (17 cases, 25%). Significant apoptosis was seen in 22 cases (33%) and marked tumor cell cannibalism was seen in 11 cases (16%). Nucleoli were prominent in 16 cases (24%). The most frequent neuroendocrine markers performed were synaptophysin and chromogranin.
Conclusions:
The most common cytomorphologic patterns seen in body cavity effusions of SCC and LCNEC were small-cell clusters with nuclear molding. However, in 51% of the cases either a predominant single-cell pattern mimicking lymphoma or large-cell clusters mimicking non-small carcinoma were noted. In our experience, effusions were the first manifestation of disease in the majority of patients diagnosed with neuroendocrine carcinoma. Therefore, familiarity with the cytomorphological spectrum of neuroendocrine carcinomas in fluid cytology may help in rapidly establishing an accurate diagnosis and in directing appropriate management.
Keywords: Cytology, effusion, neuroendocrine carcinoma, small-cell carcinoma
INTRODUCTION
Neuroendocrine carcinomas comprise a heterogeneous group of tumors that represent a spectrum of disease varying from well-differentiated tumors (e.g., carcinoid tumor) to poorly differentiated tumors, such as small-cell carcinoma (SCC) and large-cell neuroendocrine carcinoma (LCNEC). The high-grade or poorly differentiated neuroendocrine carcinomas are characterized by a more aggressive course, early metastases, and poorer prognosis.
SCCs and LCNECs are uncommon in serous body cavity effusions.[1–3] Due to this rarity, the diagnosis may be challenging for cytopathologists looking at exfoliative cytology specimens. Additional difficulties that may be encountered in fluid cytology include the overlapping morphology between non-neoplastic (lymphocytes) and similar neoplastic entities, scant cellularity, predominance of apoptosis or cellular debris, and artifactual distortion. In addition to the morphological difficulties, there may also be difficulty acquiring sufficient material for ancillary studies.
There are relatively few published reports that discuss the cytomorphologic spectrum of SCCs and LCNECs in serous effusions.[1–10] The majority of these articles are case reports or only small series of cases.[1] The goal of this study is to describe the cytomorphological features of SCCs and LCNECs in a large number of serous effusion specimens. In addition, the role of ancillary studies in establishing a diagnosis in difficult cases is discussed.
MATERIALS AND METHODS
During a period of 60 months between January 2005 and December 2009, we examined 5171 serous fluid cytology cases at our institution, of which 53 were identified as positive for SCC and 15 as positive for LCNEC [Tables 1 and 2]. These 68 cases included in this study were identified by retrospectively searching the CoPath computer database for cases that had a diagnosis of small-cell carcinoma or neuroendocrine malignancy in the final diagnosis or diagnostic comment. For each case, data regarding patient age, gender, final diagnosis, diagnostic comments, ancillary studies, previous malignant diagnoses, and all available clinical information and histologic material were recorded. At the time of final interpretation, Papanicolaou-stained, ThinPrep slides, and Diff-Quik-stained cytospins were reviewed along with two H and E-stained sections from cell block preparations. These slides were evaluated and interpreted in conjunction with the results from ancillary studies to render a final diagnosis. Immunohistochemistry was performed on deparaffinized, formalin-fixed cell block sections using a variety of different antibodies on the Ventana Benchmark® XT system (Ventana Medical Systems, Tucson, AZ), which are listed in Table 3. The following cytomorphological data were gathered from each case: cell clustering characteristics (small- and large-cell clusters), single-cell arrangements, presence of necrosis/apoptosis, presence of nucleoli, presence of molding, and the presence of tumor cell cannibalism [Table 4]. Each of these parameters was graded as none/rare (<25%), moderate (>25%r more -50%), and significant (>50%). The data were analyzed using descriptive statistics. Institutional review board approval was obtained for this study.
Table 1.
Characteristics of serous effusion cases with SCC and LCNEC (n = 68)
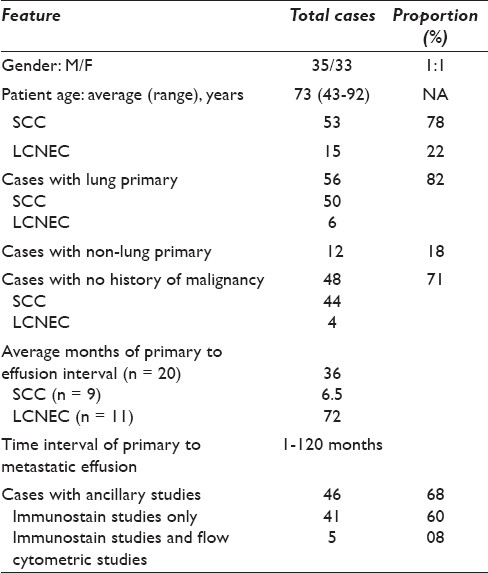
Table 2.
Distribution of cases with metastatic serous effusions (n = 68)
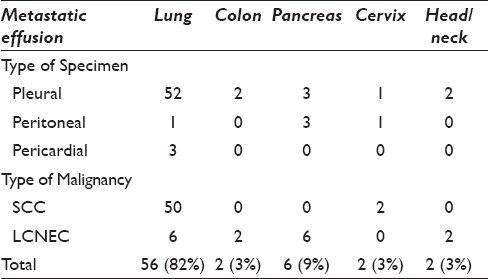
Table 3.
Antibodies, clone, host, source, and dilution of antibodies used
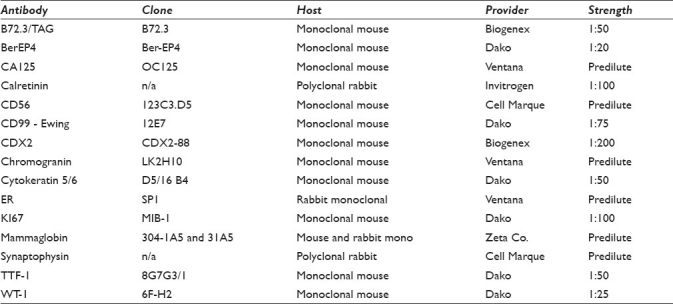
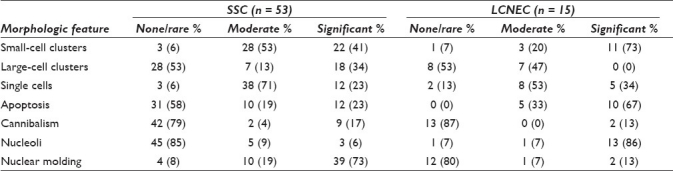
Table 4.
Cytomorphologic characteristics of malignant effusions with SCC and LCNEC
RESULTS
Clinicopathological features
We identified a total of 68 malignant effusions from 53 patients with the diagnoses of small-cell carcinoma (53 cases, 78%) and LCNEC (15 cases, 22%). The clinical features of these cases are summarized in Tables 1 and 2. The average patient age was 73 years (range 43-92 years). There were 56 cases (82%) attributed to a lung primary and 12 cases that metastasized from a nonpulmonary primary that included 6 cases from the pancreas, 2 from the colon, 2 from the cervix, and 2 cases from the head and neck region. The location of the effusions was pleural (60 cases), peritoneal (5 cases), and pericardial (3 cases). The majority of the cases had no history of malignancy of the same type (48 cases, 71%), with only 20 cases (29%) having a known diagnosis of SCC or LCNEC at clinical presentation of the effusion.
Cytopathological features
Architecturally, there were three cytomorphological predominant patterns [Figures 1–3 and Tables 4 and 5], including: (1) small-cell clusters with nuclear molding (33 cases, 49%); (2) large-cell clusters mimicking non-small-cell carcinoma (18 cases, 26%); and (3) single-cell pattern mimicking non-Hodgkin's lymphoma (17 cases, 25%). In both patterns 1 and 2, chains of small cells with nuclear molding were present.
Figure 1.
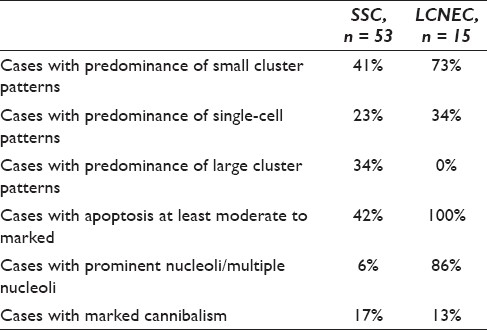
Small-cell carcinoma with a predominance of small clustering and nuclear molding in pleural fluid. The cells of small clusters display nuclear molding and scant cytoplasm (left, DQ. ×400). The nuclei are dark with a finely granular chromatin texture and inconspicuous nucleoli. Cannibalism and some cytoplasm are also seen in middle (Pap Stain, ×200). The cell block (right, H and E stain, ×400) shows a halo around a small cluster, a feature more commonly seen in fluids with adenocarcinoma
Figure 3.
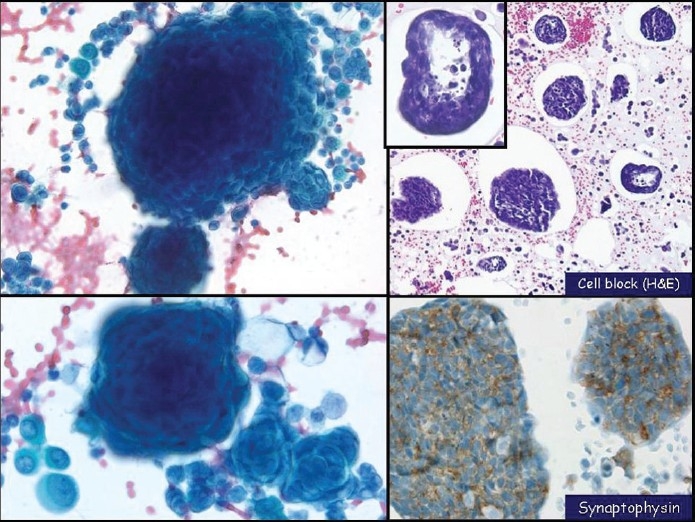
Small-cell carcinoma with a predominant single-cell pattern mimicking lymphoma in pleural fluid. The nuclei show a finely distributed granular chromatin texture and small nucleoli, left and middle (Pap stain, left ×200 and middle ×400). Chains of small cells with nuclear molding (middle, center of the image) and karyorrhexis (left) are seen. In cell block sections (right, H and E stain, ×400) tumor cells display a discohesive pattern
Table 5.
Summary of cytomorphologic spectrum of effusion with SCC and LCNEC, n = 68
In pattern 1, the cells had a small nuclear size, with a diameter approximately two to three times larger than that of small lymphocytes. The small cells displayed nuclear molding and scant cytoplasm. The nuclei were dark with a finely granular chromatin and inconspicuous nucleoli. In the cell block sections, malignant cells often showed nuclear molding, scant cytoplasm, and a halo around clusters [Figure 1]. In contrast to the background lymphocytes and mesothelial cells, the tumor cells were hyperchromatic, pleomorphic, and displayed marked nuclear contour irregularity. Tumor cell cannibalism was seen in this pattern. This pattern was seen in 69% of LCNEC cases and 42% of SCC cases [Figure 1, middle].
In pattern 2, tumor cells commonly arranged in large clusters mimicking adenocarcinoma. The crescent-shaped small cells wrapped themselves around one another (onion-ring-like morphology). Chains of small cells with nuclear molding were often present peripheral to the large clusters. In the cell block sections, malignant cells often showed an empty space (lacuna) around large clusters. Large structures showed necrosis in the center mimicking a lumen seen in adenocarcinoma [Figure 2, right]. This pattern was not seen in any of the LCNEC cases but was observed in 35% of SCC cases.
Figure 2.
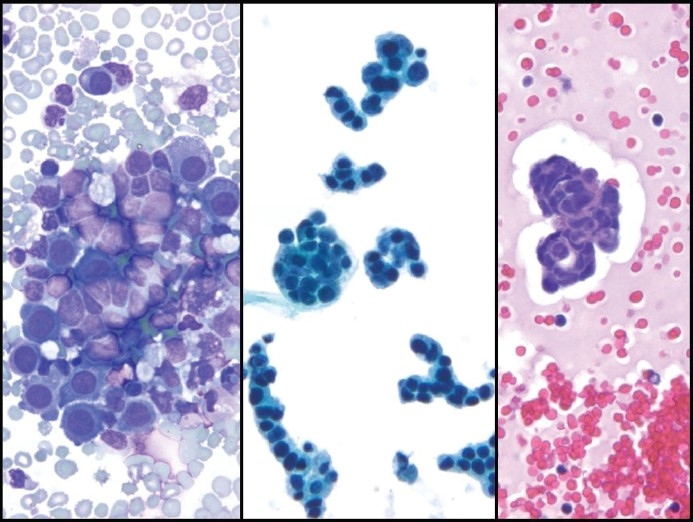
Small-cell carcinoma with a predominance of large clusters in pleural fluid. The cells have wrapped themselves around one another forming large cell clusters (left, Pap stain, ×400). Cell block (right, H and E, ×200) shows a lacuna around large clusters with hollow cores and pseudo-lumen caused by tumor cell necrosis. Synaptophysin immunostain (right lower, x400) confirms neuroendocrine differentiation in these tumor cells
In pattern 3, there was a predominant single-cell pattern that mimicked non-Hodgkin′s lymphoma in serous fluids. Chains of small tumor cells with nuclear molding were occasionally seen. The nuclear features of these tumor cells were similar to those noted in pattern 1, such as granular chromatin and inconspicuous nucleoli. Karyorrhexis [Figure 4] was more commonly seen in this pattern (13 cases, 76%). In cell block sections from these cases, tumor cells displayed a discohesive distribution mimicking lymphoma or lymphocytosis [Figure 3]. This pattern was seen in 31% of LCNEC cases and 23% of SCC cases.
Figure 4.
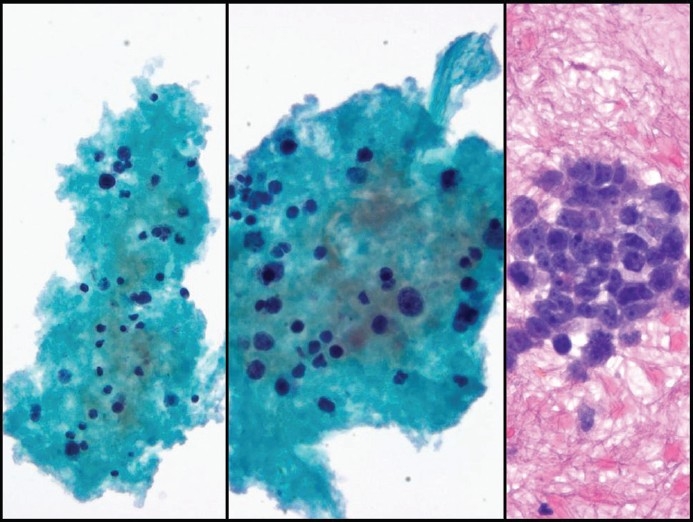
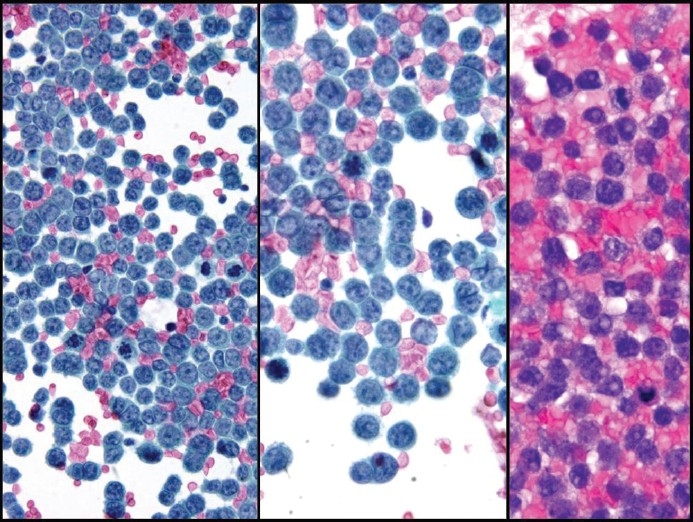
Small-cell carcinoma with marked apoptosis and small nucleoli seen in the Thin Prep (left and middle, Pap stain, ×200 and ×400, respectively) and cell block (H&E stain, × 400)
Other features such as apoptosis (karyorrhexis), nucleoli, and tumor cell cannibalism were identified in some cases of serous effusions with metastatic SCC [Figure 4–6 and Tables 4–5]. Significant apoptosis was seen in 22 cases (33%) and marked cannibalism was recorded in 11 cases (16%). Prominent nucleoli [Figures 4 and 5] were noted in 16 cases (24%), and nuclear molding was seen in 41 (60%). Ancillary studies were used in 46 cases (68%) including flow cytometric studies in 5 cases. All cases with no history of malignancy had ancillary studies to confirm the diagnosis. In most cases immunostain studies were requested in a panel including BerEp4 or B72.3; at least one mesothelial markers (calretinin, CK 5/6, or WT-1), TTFCDx-2, ER, mamaglobin, CA125, Ki67, and one or more neuroendocrine and CD56). The most frequent neuroendocrine immunostain markers performed were synaptophysin (30 cases, 82% were positive, Figures 2 and 5) and chromogranin (30 cases, 24% were positive).
Figure 6.
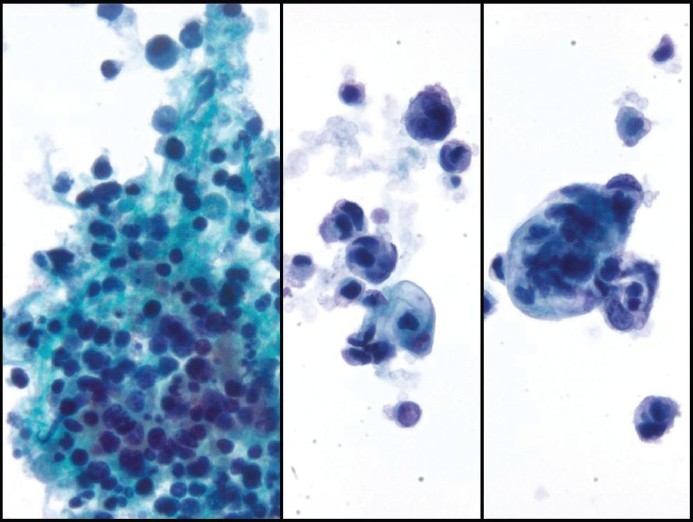
Cytomorphology of small-cell carcinoma showing marked apoptosis (left, Pap stain, ×200) and marked cannibalism (middle and right, Pap stain, ×400). [Note: Some degree of cytoplasmic vacuolization is also seen (middle and right)]
Figure 5.
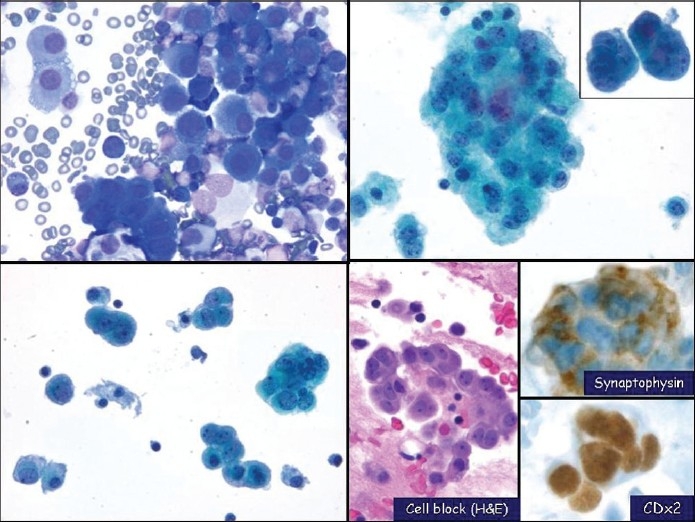
Large cell neuroendocrine carcinoma with prominent nucleoli shown in pleural fluid. The malignant cells have prominent nucleoli, nuclear enlargement and atypia. The cells also have a moderate amount of cytoplasm. (left upper, DQ cytospin, x400; left lower & right upper, Pap stain, x400; right lower, cell block H and E, x400; right lower, synaptophysin (top, x400) and CDX2 (bottom, x400) immunostains)
DISCUSSION
High-grade neuroendocrine carcinomas, such as SCC and LCNEC, are known for their aggressive behavior and widespread metastases. However, they are rarely reported in serious body cavity effusions.[1–8] For example, SCC of the lung has been reported to cause a pleural effusion in less than 3% of patients.[3] In addition, there are few published reports that discuss the cytomorphologic spectrum of SCC and LCNEC in serous effusions. To the best of our knowledge, this is the largest study to describe the cytomorphological spectrum of SCC and LCNEC in serous effusion specimens.
Our study shows that patients with SCCs or LCNECs involving serous effusions have distinct clinicopathologic features. The most common primary site where these malignancies arise is the lung (82%). Other less common sites of origin are the pancreas, cervix, colon, and head and neck. More than two-thirds of the patients in our series had no reported history of a neuroendocrine carcinoma. Of the 20 cases with a previous history of malignancy, the average interval from primary malignancy manifestation to effusion varied, with SCC having a much shorter interval compared with LCNEC.
SCC and LCNEC metastases showed a cytomorphologic spectrum within serous body cavities. Architecturally, they tend to show one of three patterns, which include a predominance of small-cell clusters (seen in both SCC and LCNEC, but more often in cases with LCNEC), a predominance of large tumor cell clusters (seen mainly in SCC), and a predominance of single tumor cells (seen in both SCC and LCNEC). About half of our cases presented predominantly with small-cell clusters. The cytological features of cases observed with a predominance of small-cell clusters have been well described in other publications.[5,6] Malignant cells that are arranged in small clusters are 2 to 2.5 times the size of lymphocytes, with scant cytoplasm. Nuclear molding, inconspicuous nucleoli, and typical salt-and-pepper nuclear chromatin are the typical features noted in SCC. Prominent nucleoli have been reported previously mainly in LCNEC. Based on these characteristic cytolomorphological, a feature along with accessory studies such as immunostains for neuroendocrine differentiation,[3,11] the diagnosis of SCC is not difficult to make.
However, when malignant neuroendocrine cells present predominantly as large-cell clusters, they are likely to be misdiagnosed as adenocarcinoma based on the cytomorphology alone. This is because small tumor cells, when aggregated into large clusters in serous effusions, develop a striking architectural similarity to adenocarcinoma. In cell block sections, malignant cells produce large clusters with a hollow center, which are characteristic cytological features seen in metastatic adenocarcinoma. In addition, a few chains of small tumor cells with nuclear molding were frequently present at the periphery of the large clusters. There are a few published articles that describe the cytological features of neuroendocrine tumors that exhibit a predominance of large-cell clusters in serous effusions. Recently, Cameron et al. reported a case of metastatic thymic well-differentiated neuroendocrine carcinoma in pleural fluid.[12] The cytological fluid preparation in this previously published case showed multiple large balls of malignant cells ranging in size from 150 to 376 μm. The large cannonball-type of clusters showed smooth community borders. They were composed of uniform small tumor cells without nucleoli. Some of the clusters also had a vague rosette-like arrangement. Large spherical aggregates in this prior case were also identified in the cell block sections. Cavitation of large clusters, however, was not present.[12] A similar feature has been described[13] in cases of mediastinal endocrine neoplasms of probable thymic origin, related to carcinoid tumor. The neoplastic clusters in these reported cases presented as large round to oval “balls” of cells, frequently observed with central necrosis and rosette formation within larger cell balls.[13] However, to make a definite diagnosis of neuroendocrine carcinoma, immunostaining with neuroendocrine markers is required.[3,11]
Approximately a quarter of our cases displayed a preponderance of single tumor cells in serous effusions. Karyorrhexis in such cases was commonly seen [Tables 4 and 5]. These cases mimicked non-Hodgkin's lymphoma in serous fluid.[14,15] Rarely, other diagnoses to entertain with a predominance of single cells in serous effusions are embryonal rhabdomyosarcoma[16] and Merkel cell carcinoma.[17] Apart from myosin immunoreactivity and the finding of myosin-type microfilaments ultrastructurally in rhabdomyosarcoma, these sarcoma cells have characteristic convoluted nuclei without prominent nuclear molding. A CK20 immunostain with a typical perinuclear dot-like staining pattern and immunopositivity with the novel Merkel cell polyomavirus (MCPyV) marker (CM2B4) can help diagnose Merkel cell carcinoma. Cases of SCC that present with a predominance of single tumor cells are not commonly seen in serous effusions. We report 17 cases of neuroendocrine tumors with a predominance of single cells. Recently, two separate groups of researchers reported a few cases of SCC also presenting mainly with single tumor cells in serous effusions. Khunamornpong et al.[4] reported two cases of SCC of the uterine cervix in serous effusions. One of these two cases showed that almost all of the tumor cells in ascites presented with a single-cell pattern that mimics malignant lymphoma. Mitotic figures and karyorrhectic bodies in this case of cervical SCC were occasionally seen.[4] Chhieng et al .[3] reported eight cases of malignant pleural effusions due to small-cell lung carcinoma. The malignant cells in this small published series by Chhieng et al. reported serous effusions characterized mainly with a predominant single-cell pattern. Rare cellular aggregates were observed only in their cell block preparations. The definite diagnosis of SCC in these cases was confirmed by immunostains using synaptophysin and chromogranin.[3] The differential diagnosis for cases in which one finds a predominance of single tumor cells in serous effusions includes SCC, lymphoma, rhabdomyosarcoma, and Merkel cell carcinoma. Immunohistochemical stains, and occasionally flow cytometry, may be crucial to definitely establish the correct diagnosis.
Finally, our data show that diagnosing LCNEC [Figure 5] is more challenging than SCC. This is due to several reasons including rare nuclear molding (only 13%), presence of a significant apoptosis (67%), and the presence of prominent nucleoli in the majority of these cases (86%). These features in LCNEC mimic poorly differentiated tumors such as adenocarcinoma. Clinical history and ancillary studies in such cases are crucial for making the proper diagnosis.
CONCLUSIONS
Our study reports 68 cases of SCCs or LCNECs metastatic to body fluids and predominantly occurred in patients without a history of malignancy. There were three predominant cytomorphological patterns seen in this series. The most common cytomorphological pattern observed was a predominance of small clustering of tumor cells. However, in approximately half of these cases, the pattern was either a single-cell pattern mimicking lymphoma or a large clustering pattern mimicking non-small carcinoma. Therefore, knowledge of the cytomorphological spectrum caused by neuroendocrine carcinomas in fluids may help in establishing timely and accurate diagnoses, which can help the management of afflicted patients.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE. All authors are responsible for the conception of this study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) at University of Pittsburgh Medical Center (UPMC), Conemaugh Memorial Medical Center, Johnstown, PA 15905, and the Department of Pathology, Fletcher Allen Health Care, Burlington, VT 05401, USA. The authors maintain relevant documentation in this respect.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model(authors are blinded for reviewers and reviewers are blinded for authors)through automatic online system.
Footnotes
Available FREE in open access from: http://www.cytojournal.com/text.asp?2011/8/1/18/86816.
Contributor Information
Walid E. Khalbuss, Email: khalbussw2@upmc.edu.
Huaitao Yang, Email: hyang@conemaugh.org.
Qian Lian, Email: lqian@conemaugh.org.
Abdelmonem Elhosseiny, Email: A-Abdel.Elhosseiny@vtmednet.org.
Liron Pantanowitz, Email: pantanowitzl@upmc.edu.
Sara E Monaco, Email: monacose@upmc.edu.
REFERENCES
- 1.Domagała-Kulawik J, Górnicka B, Krenke R, Mich S, Chazan R. The value of cytological diagnosis of small cell lung carcinoma. Pneumonol Alergol Pol. 2010;3:203–10. [PubMed] [Google Scholar]
- 2.Awasthi A, Gupta N, Srinivasan R, Nijhawan R, Rajwanshi A. Cytopathological spectrum of unusual malignant pleural effusions at a tertiary care centre in north India. Cytopathology. 2007;18:28–32. doi: 10.1111/j.1365-2303.2007.00382.x. [DOI] [PubMed] [Google Scholar]
- 3.Chhieng DC, Ko EC, Yee HT, Shultz JJ, Dorvault CC, Eltoum IA. Malignant pleural effusions due to small-cell lung carcinoma: a cytologic and immunocytochemical study. Diagn Cytopathol. 2001;25:356–60. doi: 10.1002/dc.10011. [DOI] [PubMed] [Google Scholar]
- 4.Khunamornpong S, Siriaunkgul S, Suprasert P. Cytology of small-cell carcinoma of the uterine cervix in serous effusion: a report on two cases. Diagn Cytopathol. 2001;24:253–5. doi: 10.1002/dc.1055. [DOI] [PubMed] [Google Scholar]
- 5.Salhadin A, Nasiell M, Nasiell K, Silfverswärd C, Hjerpe A, Wadäs AM, et al. The unique cytologic picture of oat cell carcinoma in effusions. Acta Cytol. 1976;20:298–302. [PubMed] [Google Scholar]
- 6.Spriggs AI, Boddington MM. Oat-cell bronchial carcinoma.Identification of cells in pleural fluid. Acta Cytol. 1976;20:525–9. [PubMed] [Google Scholar]
- 7.Masuko H, Satoh H, Ohara G, Anami Y, Noguchi M, Ohtsuka M. Pleural small cell carcinoma. Lung Cancer. 2006;53:391–2. doi: 10.1016/j.lungcan.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Klee H, Vestring T, Bittmann I. Pleural metastases of a typical bronchial carcinoid 7 years after lobectomy. Pneumologie. 2008;62:607–10. doi: 10.1055/s-2008-1038226. [DOI] [PubMed] [Google Scholar]
- 9.Shidham VB. Metastatic Carcinoma in Effusion. In: Shidham VB, Atkinson BF, editors. ‘Cytopathologic Diagnosis of Serous Fluids’. Philadelphia, PA: Elsevier W.B. Saunders Company; 2007. pp. 115–45. [Google Scholar]
- 10.Selvaggi SM. Small-cell carcinoma of the ovary in peritoneal fluid. Diagn Cytopathol. 1994;11:266–70. doi: 10.1002/dc.2840110314. [DOI] [PubMed] [Google Scholar]
- 11.Tamiolakis D, Papadopoulos N, Cheva A, Lambropoulou M, Kotini A, Mikroulis D, et al. Immunocytochemical profile of malignant pleural effusions of small-cell lung cancer. Minerva Med. 2002;93:479–83. [PubMed] [Google Scholar]
- 12.Cameron SE, Alsharif M, McKeon D, Pambuccian SE. Cytology of metastatic thymic well-differentiated neuroendocrine carcinoma (thymic carcinoid) in pleural fluid: report of a case. Diagn Cytopathol. 2008;36:333–7. doi: 10.1002/dc.20810. [DOI] [PubMed] [Google Scholar]
- 13.Rosai J, Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor.Clinicopathologic study of 8 cases. Cancer. 1972;29:1061–74. doi: 10.1002/1097-0142(197204)29:4<1061::aid-cncr2820290456>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Brimo F, Popradi G, Michel RP, Auger M. Primary effusion lymphoma involving three body cavities. CytoJournal. 2009;6:21. doi: 10.4103/1742-6413.56361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335–47. doi: 10.1002/dc.20432. [DOI] [PubMed] [Google Scholar]
- 16.Thompson KS, Jensen JD, Bhoopalam N, Reyes CV. Pleural effusion cytology of embryonal rhabdomyosarcoma. Diagn Cytopathol. 1997;16:270–3. doi: 10.1002/(sici)1097-0339(199703)16:3<270::aid-dc16>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Payne MM, Rader AE, McCarthy DM, Rodgers WH. Merkel cell carcinoma in a malignant pleural effusion: case report. Cytojournal. 2004;1:5. doi: 10.1186/1742-6413-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


