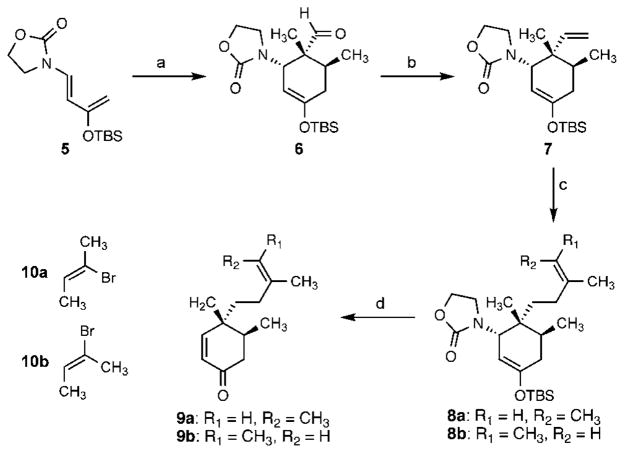
Scheme 2.
Diels–Alder-mediated synthesis of enones 9a,b. Conditions: (a) tiglaldehyde, toluene, 120 °C, 80%; (b) Ph3PCH3Br, n-BuLi, THF, 0°C, 67%; (c) 8a: 9-BBN, THF, reflux; then 10 mol% PdCl2(dppf), aq. K3PO4, 10a, DMF, 50 °C; 8b: 9-BBN, THF, reflux; then 10 mol% PdCl2(dppf), aq. K3PO4, 10b, DMF, 50 °C; (d) 9a: TBAF, THF, 86% (2 steps); 9b: TBAF, THF, 100% (2 steps). 9-BBN = 9-bor-abicyclo(3.3.1)nonane; dppf =1,1′-bis(diphenylphosphino)ferrocene; TBAF = tetra-n-butylammonium fluoride.