Scheme 4.
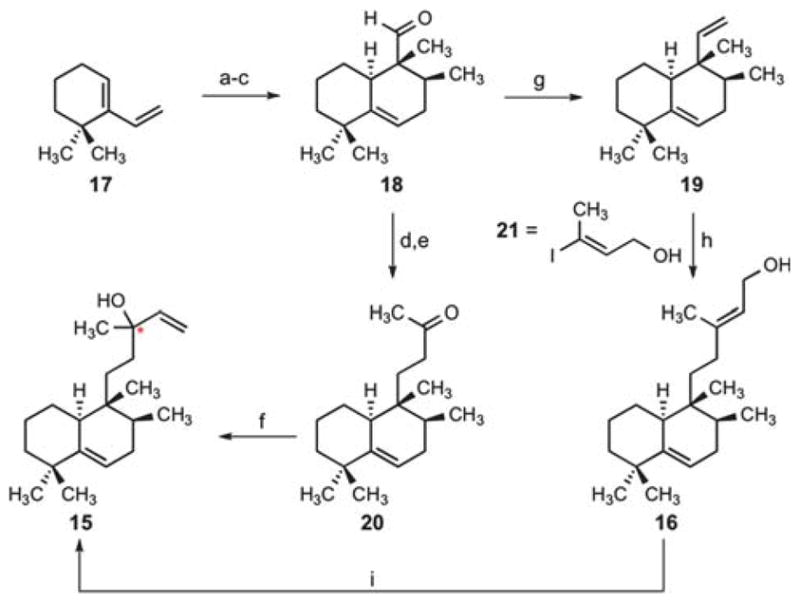
Syntheses of nosyberkol and tuberculosinol via an exo-selective Diels–Alder reaction. Conditions: (a) ethyl tiglate, neat, 160 °C, 71% (2:1 exo:endo); (b) LiAlH4, THF, 40 °C, 56% (+24% endo isomer); (c) SO3·pyridine, NEt3, CH2Cl2–DMSO, 0 °C, 86%; (d) acetone, NaHMDS, THF, −78 → 23 °C, 87%; (e) 10 mol% Rh(PPh3)3Cl, HSiEt3, CH2Cl2, 40 °C, 83%; (f) vinylmagnesium bromide, THF, 0 °C, 93% (1.5:1 dr at the starred (*) carbon); (g) Ph3PCH3Br, KHMDS, 0 °C, THF, 91%; (h) 9-BBN, THF, 80 °C; then 10 mol% PdCl2(dppf), Ph3As, Cs2CO3, 21, DMF, 73%; (i) 20 mol% CuCl2, acetone, 20% (dr = 1:1 at the starred (*) carbon). 9-BBN =9-borabicyclo(3.3.1)nonane; dppf = 1,1′-bis(diphenylphosphino)ferrocene.
