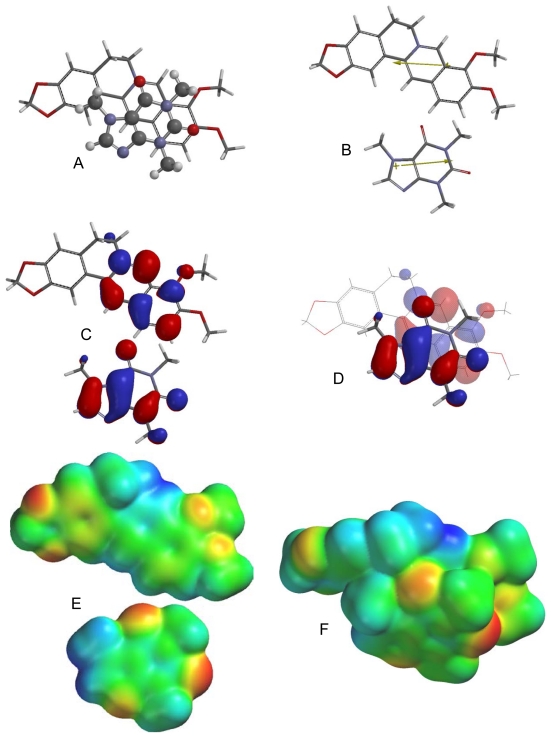
Fig. 2.
Lowest-energy orientation of the π–π complex between berberine and caffeine. (A) Face-to face orientation of caffeine (ball and spoke model) in its lowest-energy orientation with berberine (tube model). (B) Molecular dipoles of berberine (top) and caffeine (bottom). (C) LUMO of berberine (top) and HOMO of caffeine (bottom). (D) Frontier molecular orbital overlap of caffeine with berberine in the lowest-energy orientation. (E) Electrostatic potential maps of berberine (top) and caffeine (bottom). (F) Electrostatic potential map of the lowest-energy π–π complex between berberine and caffeine.